emmy
- 36
- 0
I recently ran a baylis-hillman reaction of 2nitrobenzaldehyde and acrylonitrile (DABCO catalyst) and after my reaction had gone to completion (determined by tlc) i extracted with chloroform/washed with brine, dried, filtered, rotovaped etc etc
I ran a crude NMR and determined I did have my desired product.
I ran a silica pipette column with %anhydrous diethyl ether/hexanes and took HNMR again, it indicated that my carbonyl had become a hydroxyl group.
The only thing I can figure is that the silica gel in the column protonated my carbonyl. I know that silica gel is somewhat acidic...does anyone know if this would likely occur or what else could have occurred? )':
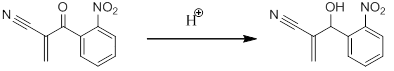
MBH product on left, my product after column on right
I ran a crude NMR and determined I did have my desired product.
I ran a silica pipette column with %anhydrous diethyl ether/hexanes and took HNMR again, it indicated that my carbonyl had become a hydroxyl group.
The only thing I can figure is that the silica gel in the column protonated my carbonyl. I know that silica gel is somewhat acidic...does anyone know if this would likely occur or what else could have occurred? )':
MBH product on left, my product after column on right