ifihadsomebacon
- 4
- 0
Going from:
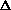 E = q + w
E = q + w
To:
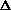 H =
H =
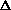 E +
E +
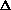 (PV)
(PV)
I'm confused as to why you add the product of the pressure and volume of the system to the internal energy to get enthalpy. Is it just because "that's what enthalpy is defined as"? I think I understand that when holding pressure constant, the
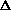 (PV) becomes P
(PV) becomes P
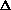 V and cancels with the w = P
V and cancels with the w = P
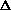 V from
V from
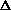 E, giving
E, giving
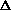 H = q. If pressure is not constant, does that mean
H = q. If pressure is not constant, does that mean
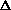 H = q + w +
H = q + w +
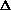 (PV) or
(PV) or
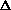 H = q + P
H = q + P
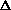 V +
V +
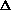 (PV) is true? What does this mean, practically? I just am wondering why are there two "PV" expressions in the first place? What even is
(PV) is true? What does this mean, practically? I just am wondering why are there two "PV" expressions in the first place? What even is
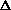 (PV)? It doesn't make sense to me like P
(PV)? It doesn't make sense to me like P
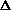 V, what about
V, what about
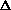 (PV) makes it so it can be added on initially?
(PV) makes it so it can be added on initially?
I know I asked a lot of questions, I'm just trying to make my confusion clear. (Ha)
To:
I'm confused as to why you add the product of the pressure and volume of the system to the internal energy to get enthalpy. Is it just because "that's what enthalpy is defined as"? I think I understand that when holding pressure constant, the
I know I asked a lot of questions, I'm just trying to make my confusion clear. (Ha)
Attachments
-
 delta.gif63 bytes · Views: 2,968
delta.gif63 bytes · Views: 2,968 -
 delta.gif63 bytes · Views: 3,120
delta.gif63 bytes · Views: 3,120 -
 delta.gif63 bytes · Views: 2,947
delta.gif63 bytes · Views: 2,947 -
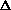 delta.gif63 bytes · Views: 3,040
delta.gif63 bytes · Views: 3,040 -
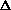 delta.gif63 bytes · Views: 2,884
delta.gif63 bytes · Views: 2,884 -
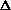 delta.gif63 bytes · Views: 2,743
delta.gif63 bytes · Views: 2,743 -
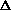 delta.gif63 bytes · Views: 2,641
delta.gif63 bytes · Views: 2,641 -
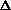 delta.gif63 bytes · Views: 2,728
delta.gif63 bytes · Views: 2,728 -
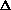 delta.gif63 bytes · Views: 2,742
delta.gif63 bytes · Views: 2,742 -
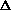 delta.gif63 bytes · Views: 2,736
delta.gif63 bytes · Views: 2,736 -
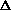 delta.gif63 bytes · Views: 2,768
delta.gif63 bytes · Views: 2,768 -
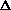 delta.gif63 bytes · Views: 2,601
delta.gif63 bytes · Views: 2,601 -
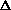 delta.gif63 bytes · Views: 2,764
delta.gif63 bytes · Views: 2,764 -
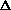 delta.gif63 bytes · Views: 2,729
delta.gif63 bytes · Views: 2,729 -
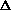 delta.gif63 bytes · Views: 2,631
delta.gif63 bytes · Views: 2,631 -
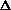 delta.gif63 bytes · Views: 2,750
delta.gif63 bytes · Views: 2,750 -
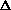 delta.gif63 bytes · Views: 2,784
delta.gif63 bytes · Views: 2,784