SUMMARY
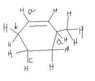
The discussion focuses on the stability of allylic carbanions formed from the cleavage of C-H bonds. Specifically, it identifies that cleavage of option (b) results in a carbanion that is stabilized by resonance, making it more stable than the vinylic carbanion produced from option (a). The consensus is that the allylic carbanions formed from positions 'b' and 'd' are the most stable, with option 'b' being the preferred choice due to its resonance stabilization.
PREREQUISITES
- Understanding of carbanion formation and stability
- Knowledge of resonance structures in organic chemistry
- Familiarity with C-H bond cleavage mechanisms
- Basic concepts of allylic and vinylic positions in organic compounds
NEXT STEPS
- Research the mechanisms of carbanion stability and resonance effects
- Study the differences between allylic and vinylic carbanions
- Explore examples of C-H bond cleavage in organic reactions
- Learn about the implications of carbanion stability in synthetic organic chemistry
USEFUL FOR
Chemistry students, organic chemists, and anyone studying reaction mechanisms involving carbanions and their stability.