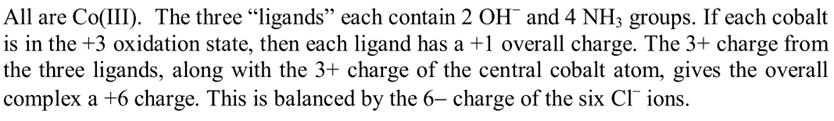SUMMARY
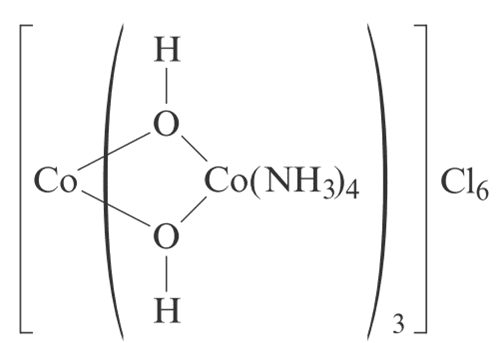
The oxidation state of cobalt in the discussed complex is +6 for the central cobalt atom, surrounded by six oxygen atoms, while the other cobalt atoms exhibit an oxidation state of +2 due to their coordination with two oxygen atoms and NH3 ligands. The discussion emphasizes that oxidation states are merely accounting tools and do not reflect measurable properties of atoms. Assigning oxidation states in large complexes is often impractical, as these atoms are interconnected within molecular orbitals, complicating the concept of charge distribution. The conversation also highlights the importance of considering charge balance in complex ions, as seen in the example of Fe3O4.
PREREQUISITES
- Understanding of oxidation states in coordination chemistry
- Familiarity with molecular orbital theory
- Knowledge of spectroscopic methods for measuring charge density
- Basic principles of electron bookkeeping in chemistry
NEXT STEPS
- Research "Coordination Chemistry and Oxidation States" for deeper insights
- Study "Molecular Orbital Theory" to understand bonding in complexes
- Explore "Charge Density Measurement Techniques" in spectroscopy
- Investigate "Fe3O4 Structure and Properties" for practical applications of oxidation states
USEFUL FOR
Chemistry students, theoretical chemists, and professionals in coordination chemistry seeking to deepen their understanding of oxidation states and molecular interactions in complex ions.