phantomvommand
- 287
- 39
This is the Two-Balloon Experiment: https://en.wikipedia.org/wiki/Two-balloon_experiment#cite_note-MW78-1
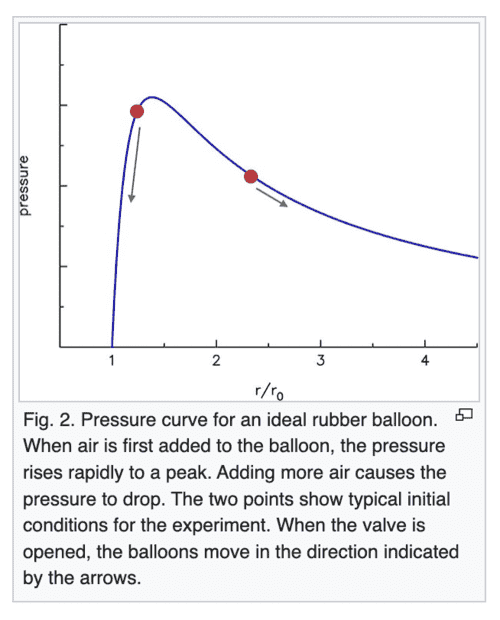
The claim on Wikipedia which I am a little confused over is that when 2 balloons (at the 2 red points) are connected via a tube, the smaller balloon at a higher pressure would push air into the larger balloon. Eventually, the pressure should become equal in both balloons.
My first question is this:
According to the article, the "pressure" that must become equal eventually is the pressure in the rubber of the balloon. However, shouldn't it be the pressure of the air in the 2 balloons?
Assuming that equal rubber pressure implies equal air pressure,
considering the system as a whole:
P1V1 + P2V2 must be equal to P(V1 + V2), where P is the final equal air pressure. However, P1 > P and P2 > P, so the equality cannot hold. (LHS > RHS)
I understand that I have not accounted for the changes in energy when the rubber balloons acquire a more stable state (at lower pressure). But if this were accounted for, we can see that:
U1 + U2 + Rubber energy initial = U3 + Rubber energy final, where U1, U2, and U3 are the internal energies of the air in the small balloon, bigger balloon and final state.
Since U1, U2, U3 are analogous to P1V1, P1V2, and P(V1+V2), and rubber energy final > initial, LHS > RHS.
Where have I gone wrong?
The claim on Wikipedia which I am a little confused over is that when 2 balloons (at the 2 red points) are connected via a tube, the smaller balloon at a higher pressure would push air into the larger balloon. Eventually, the pressure should become equal in both balloons.
My first question is this:
According to the article, the "pressure" that must become equal eventually is the pressure in the rubber of the balloon. However, shouldn't it be the pressure of the air in the 2 balloons?
Assuming that equal rubber pressure implies equal air pressure,
considering the system as a whole:
P1V1 + P2V2 must be equal to P(V1 + V2), where P is the final equal air pressure. However, P1 > P and P2 > P, so the equality cannot hold. (LHS > RHS)
I understand that I have not accounted for the changes in energy when the rubber balloons acquire a more stable state (at lower pressure). But if this were accounted for, we can see that:
U1 + U2 + Rubber energy initial = U3 + Rubber energy final, where U1, U2, and U3 are the internal energies of the air in the small balloon, bigger balloon and final state.
Since U1, U2, U3 are analogous to P1V1, P1V2, and P(V1+V2), and rubber energy final > initial, LHS > RHS.
Where have I gone wrong?