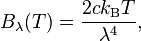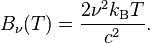SUMMARY
The discussion centers on converting between wavelength (λ) and frequency (ν) in the context of blackbody radiation, specifically using the Rayleigh-Jeans Law. The relationship is established through the equation ν = c/λ, leading to the expressions for spectral radiance: Bν = (2kBTν²/c²) and Bλ = (2ckBT/λ⁴). The conversation highlights the limitations of the Rayleigh-Jeans Law at short wavelengths, known as the ultraviolet catastrophe, and discusses the measurement of spectral radiance using different equipment for Bλ and Bν.
PREREQUISITES
- Understanding of blackbody radiation concepts
- Familiarity with the Rayleigh-Jeans Law
- Basic knowledge of spectral radiance equations
- Knowledge of the relationship between wavelength and frequency
NEXT STEPS
- Research the Planck's Law of blackbody radiation
- Learn about the ultraviolet catastrophe and its implications
- Explore the equipment used for measuring spectral radiance (Bλ and Bν)
- Study the derivation of the Rayleigh-Jeans Law and its limitations
USEFUL FOR
Physicists, engineers, and students studying thermodynamics and electromagnetic radiation, particularly those interested in blackbody radiation and its measurement techniques.