AdityaDev
- 527
- 33
I am confused with the electron configuration of central atoms in complex salts eg. ##[Fe(CN)_6]^{2-}## configuration just Fe atom is
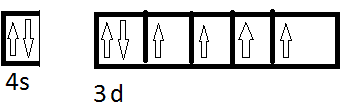
Now the complex is low spin so the configuration becomes,
Now we have 6CN. where will they donate their electrons? Will the donate electrons in the remaining ##e_g## to form ##d^nsp^m## or will they donate electrons in the 4s, 4p and 4d to form ##sp^nd^m## or will they have some other configuration? Can you give some exceptional cases (like for Pt all ligands are strong field and most are square planar)
I have read Crystal Field Theory and VSEPR theory. I also know Jahn-teller distorsion and Crystal field splitting.Also, the oxidation number of Fe is +4.
So will the electrons first be removed first from 4s before 3d?Is this true for all cases?
.
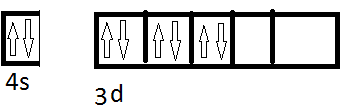
Now the complex is low spin so the configuration becomes,
Now we have 6CN. where will they donate their electrons? Will the donate electrons in the remaining ##e_g## to form ##d^nsp^m## or will they donate electrons in the 4s, 4p and 4d to form ##sp^nd^m## or will they have some other configuration? Can you give some exceptional cases (like for Pt all ligands are strong field and most are square planar)
I have read Crystal Field Theory and VSEPR theory. I also know Jahn-teller distorsion and Crystal field splitting.Also, the oxidation number of Fe is +4.
So will the electrons first be removed first from 4s before 3d?Is this true for all cases?
.
Last edited by a moderator: