SUMMARY
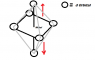
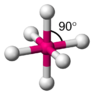
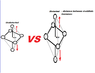
The discussion centers on the color intensity of copper nitrate (Cu(NO3)2) and its relationship to the octahedral structure and ligand field theory. Participants explain that the blue color arises from the Ligand Field Stabilization Energy (LFSE) changes due to electron redistribution in the d orbitals, specifically the eg and t2g orbitals. The intensity of the blue color is influenced by the distance between the d orbitals, with greater distances leading to higher energy transitions and more intense colors. Additionally, hydration plays a crucial role, as the hydrated form of copper nitrate exhibits the characteristic blue color.
PREREQUISITES
- Understanding of octahedral coordination complexes
- Familiarity with Ligand Field Theory and Ligand Field Stabilization Energy (LFSE)
- Knowledge of d-d transitions in transition metal complexes
- Basic concepts of vibronic coupling and its effects on electronic transitions
NEXT STEPS
- Study the principles of Crystal Field Theory and its application to transition metal complexes
- Learn about the Jahn-Teller effect and its influence on octahedral complexes
- Research the impact of hydration on the properties of metal complexes, specifically copper compounds
- Explore Tanabe-Sugano diagrams for a deeper understanding of d-orbital splitting and transitions
USEFUL FOR
Chemistry students, inorganic chemists, and anyone interested in the optical properties of transition metal complexes, particularly those studying copper compounds and their colorimetric behavior.