- #1
Bolter
- 262
- 31
- Homework Statement
- Calculate the density of air
- Relevant Equations
- PV = nRT
How would I tackle a problem like this?
I made a start by writing down the ideal gas equation and then done some manipulation on both sides to get the density expression of the ideal gas.
I'm not sure if this is what the question wants as I'm dealing with 2 different types of gases in the same atmosphere.
My density formula that I have obtained only applies to find the density of one type of gas?
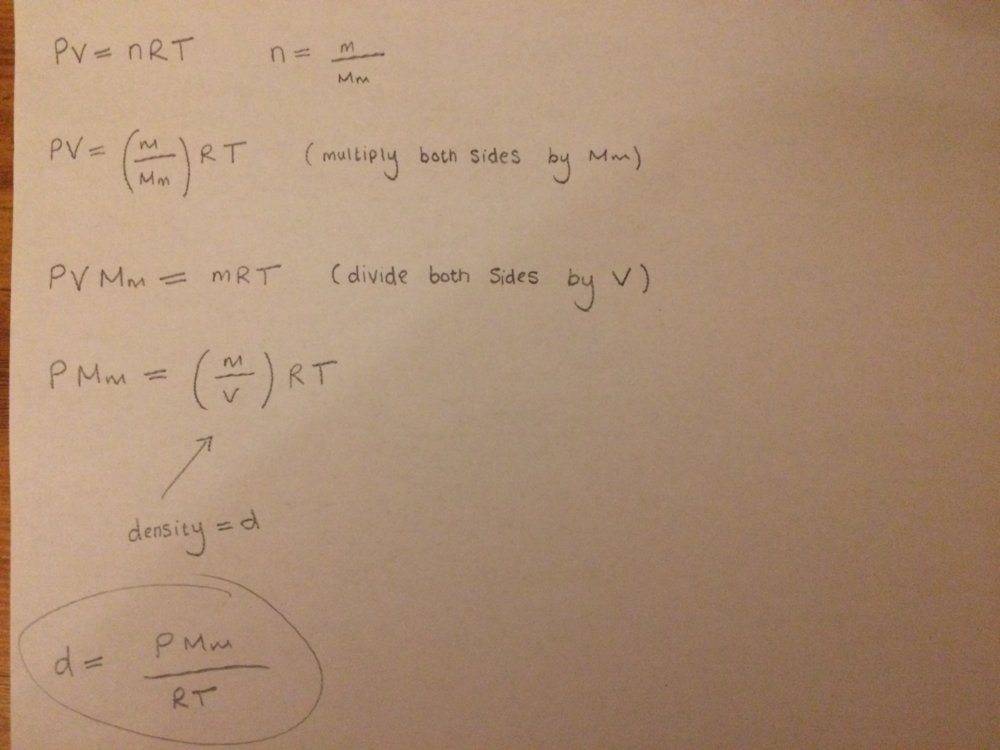
Also is there a typo in the question itself? Should it read "Determine the density of air at 25 degrees at one atmosphere pressure" instead of "Determine the density of air at 25 degrees and one atmosphere pressure"?
Any help would be grateful!
I made a start by writing down the ideal gas equation and then done some manipulation on both sides to get the density expression of the ideal gas.
I'm not sure if this is what the question wants as I'm dealing with 2 different types of gases in the same atmosphere.
My density formula that I have obtained only applies to find the density of one type of gas?
Also is there a typo in the question itself? Should it read "Determine the density of air at 25 degrees at one atmosphere pressure" instead of "Determine the density of air at 25 degrees and one atmosphere pressure"?
Any help would be grateful!