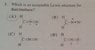SUMMARY
The acceptable Lewis structure for Diazomethane is structure B, as confirmed by the discussion participants. Structures C and D are incorrect resonance forms. The reasoning provided highlights that nitrogen cannot have four bonds and a lone pair, which aligns with the octet rule. This conclusion is based on the electronegativity of nitrogen compared to carbon, emphasizing the stability of formal charges in resonance structures.
PREREQUISITES
- Understanding of Lewis structures and resonance forms
- Familiarity with the octet rule in chemistry
- Knowledge of formal charge calculations
- Basic concepts of electronegativity and its implications in molecular stability
NEXT STEPS
- Study the octet rule and its exceptions in molecular structures
- Learn about resonance structures and their significance in chemical bonding
- Explore formal charge calculations for various molecular configurations
- Investigate the role of electronegativity in determining molecular stability
USEFUL FOR
Chemistry students, educators, and anyone studying molecular structure and bonding, particularly in organic chemistry.