- #1
bonfire09
- 249
- 0
dienophile and diene help?? organic chemistry
We never really covered how to do diels alder reactions and I am having trouble how
to do these. Here is a photo of the lab page with the questions. I just need help
with 2a-e and 3b-3d. Thanks.
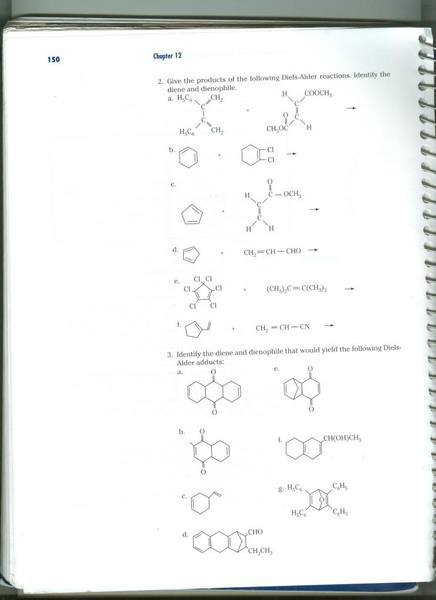
We never really covered how to do diels alder reactions and I am having trouble how
to do these. Here is a photo of the lab page with the questions. I just need help
with 2a-e and 3b-3d. Thanks.





