nobahar
- 482
- 2
Hello,
I have found this chemistry equipment, but I cannot determine what it is for. I have a suspicion it is for measuring boiling point temperature elevation.
I have included a drawing of how I think it should go together. It consists of a conical flask connected to a graduated boiling tube (D) (for volume); the conical flask has a two-holed bung: one aperture contains a delivery tube (B) connected to the boiling tube (there are small holes in the delivery tube within the boiling tube, and the other has a glass cylinder passing vertically upwards, which is angled at the bottom (A; this might be to do with maintaining atmospheric pressure?). The boiling tube has a bulb on the upper portion. The boiling tube is contained within a larger glass container (C). There is an aperture in the boiling tube opening into the larger container (E). The larger container has a spout at the bottom.
So the liquid in the conical flask is heated and passes through E and condenses in the boiling tube (D). I am not sure if the delivery part, E, has to be lower, so that as the liquid condenses the delivery tube becomes submerged in the liquid. Then I am guessing the liquid that condenses in the boiling tube will at some point be hot enough to pass from the boiling tube, through aperture E into the larger container (C). Quite what is happening, I am not sure...
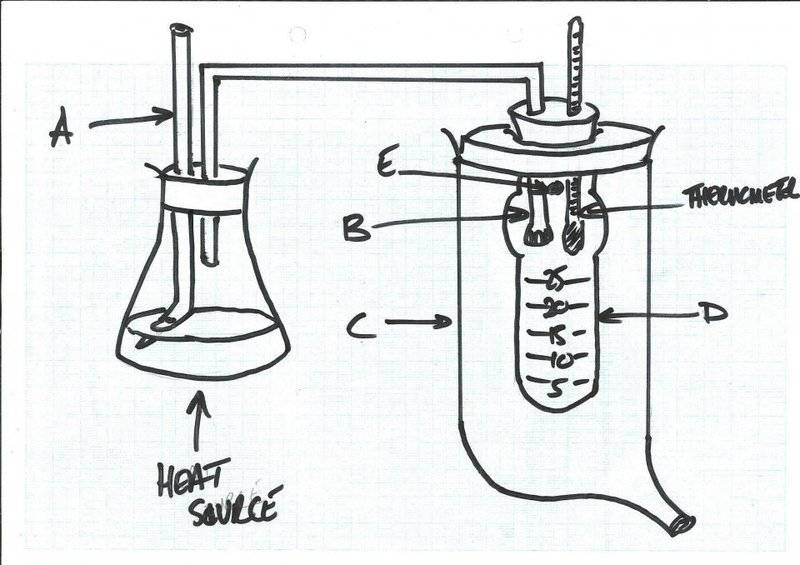
I have found this chemistry equipment, but I cannot determine what it is for. I have a suspicion it is for measuring boiling point temperature elevation.
I have included a drawing of how I think it should go together. It consists of a conical flask connected to a graduated boiling tube (D) (for volume); the conical flask has a two-holed bung: one aperture contains a delivery tube (B) connected to the boiling tube (there are small holes in the delivery tube within the boiling tube, and the other has a glass cylinder passing vertically upwards, which is angled at the bottom (A; this might be to do with maintaining atmospheric pressure?). The boiling tube has a bulb on the upper portion. The boiling tube is contained within a larger glass container (C). There is an aperture in the boiling tube opening into the larger container (E). The larger container has a spout at the bottom.
So the liquid in the conical flask is heated and passes through E and condenses in the boiling tube (D). I am not sure if the delivery part, E, has to be lower, so that as the liquid condenses the delivery tube becomes submerged in the liquid. Then I am guessing the liquid that condenses in the boiling tube will at some point be hot enough to pass from the boiling tube, through aperture E into the larger container (C). Quite what is happening, I am not sure...