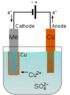Electrolysis of Water: How Does the Electric Field Affect Covalent Bonds?
- Context: Undergrad
- Thread starter erocored
- Start date
-
- Tags
- Electrolysis Work
Click For Summary
Discussion Overview
The discussion revolves around the electrolysis of water and the role of electric fields in breaking bonds, particularly focusing on the dissociation of CuSO4 in water and the behavior of electrodes during the process. Participants explore theoretical and practical aspects of electrolysis, including the movement of ions and the nature of covalent bonds.
Discussion Character
- Exploratory
- Technical explanation
- Debate/contested
- Mathematical reasoning
Main Points Raised
- Some participants question what specifically breaks bonds in CuSO4 and why the right electrode becomes positively charged during electrolysis.
- Others explain that CuSO4 dissociates in water, with Cu++ and SO4-- ions becoming stabilized by hydration, which requires energy to separate them.
- A participant suggests that the electric field moves ions apart rather than breaking bonds directly, while also noting that a strong enough electric field can break covalent bonds in other molecules, such as water.
- Some participants discuss the complexity of electrolysis, mentioning the role of the Fermi level in electron transfer and the kinetic processes involved.
- There are differing views on whether the electric field directly splits covalent bonds, with some arguing that it does not while others suggest it can under certain conditions.
- One participant emphasizes the importance of understanding the self-ionization of water and the role of hydronium ions in the electrolysis process.
Areas of Agreement / Disagreement
Participants express multiple competing views regarding the mechanisms of electrolysis and the role of electric fields in bond breaking. The discussion remains unresolved, with no consensus on whether the electric field directly affects covalent bonds.
Contextual Notes
Participants highlight the complexity of the electrolysis process, including the need for electrolytes to enhance efficiency and the nuances of ion migration and bond interactions. There are references to specific chemical equations and mechanisms that remain open to interpretation.
Similar threads
- · Replies 5 ·
- · Replies 3 ·
- · Replies 8 ·
- · Replies 20 ·
- · Replies 5 ·
- · Replies 3 ·
- · Replies 1 ·
- · Replies 14 ·
- · Replies 4 ·
- · Replies 3 ·