Discussion Overview
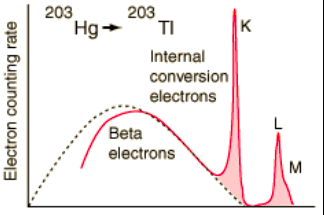
The discussion revolves around the frequency of K-shell electrons being emitted during internal conversion compared to L or M-shell electrons. Participants explore the underlying probabilities and mechanisms involved in this process, touching on concepts from quantum mechanics and atomic structure.
Discussion Character
- Exploratory
- Technical explanation
- Debate/contested
Main Points Raised
- One participant questions why K-shell electrons are emitted more frequently than those from higher shells, suggesting that it might be easier for gamma particles to dislodge less tightly bound electrons.
- Another participant asserts that the probability of an electron being found within the nucleus decreases with higher shells, indicating that K electrons are more likely to be involved in internal conversion.
- A participant challenges the phrasing regarding electrons being "within the nucleus," clarifying that electrons occupy atomic orbitals outside the nucleus.
- In response, another participant argues that the wave function of electrons has a non-zero probability of being found at the nucleus, suggesting that K-shell electrons have a higher likelihood of being involved in internal conversion due to their wave function characteristics.
- A later reply provides an example from external resources, noting that the probability of an electron being found at the nucleus is smaller for excited states compared to ground states.
- One participant encourages others to compare the radius of nuclei undergoing internal conversion with the Bohr radius to understand the relevance of the discussed graphs.
Areas of Agreement / Disagreement
Participants express differing views on the interpretation of electron probabilities and their relation to internal conversion, indicating that multiple competing perspectives remain without a clear consensus.
Contextual Notes
Some participants' claims depend on the definitions of electron positions and the nature of wave functions, which may not be fully resolved in the discussion.