- #1
GTrax
- 156
- 10
- TL;DR Summary
- If electrons in elements are energized by an incoming gamma photon, what happens to excess energy? Can a very energetic photon affect more than one atom?
Tentatively, I ask in this forum for a qualitative pointer to what end effects one might expect when a gamma energy photon energizes an atom of a substance, and causes fluorescence. It relates to a practical endeavour about using a PIN diode as an X-ray detector, where the device considered happens to generate currents from X-ray photons having energies in a useful range from about 1.5keV to about 200keV.
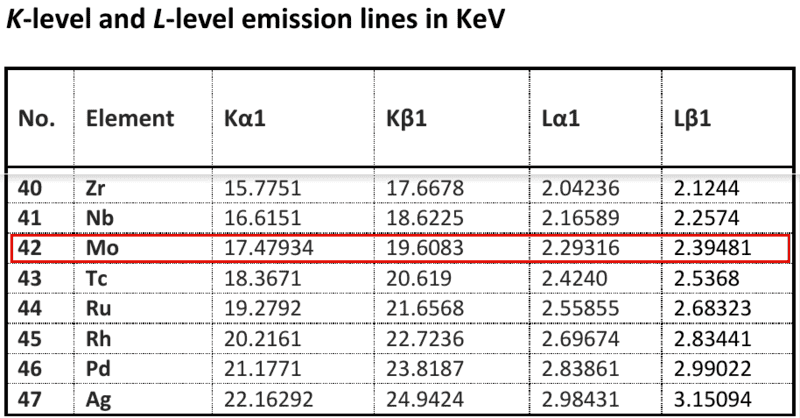
This leads to a some questions. I use, as example, (say) molybdenum, for which I have data in a fragment from a table of X-ray emission energies.
1. Will an incoming (gamma) photon of energy a bit more than the total required to excite all the shell electrons always get to cause fluorescence at all the wavelengths corresponding? Is there some probability that only one or two might glow?
2. For an incoming photon of (say) enough energy to excite all the electrons twice over, what happens to the excess energy? Can it ever go on to excite electrons in another atom?
3. Is there any forced order in the probabilities of electrons shells accepting energy? For example, do the L-shell electrons get raised by their quanta first, such that a photon with insufficient energy to affect the K-shell candidates, will still get glows from L-shell probable action? Is it take-up in order of energy, lowest first?
4. Is there some probability that a 20keV photon might excite a single Kβ1 ray from molybdenum at 17.48keV, without any others happening?
5.. Are there probabilities that an incoming photon, of enough energy to do something, might fail to do anything at all?
6.. Once a photon has caused some electron excitations, is it then "used up"? Does the excess then simply raise the temperature of the atom?
Please forgive the very basic nature of these questions. I do understand they may be inappropriate in this forum, but they do also fall outside the range of most other electronics design groups.
This leads to a some questions. I use, as example, (say) molybdenum, for which I have data in a fragment from a table of X-ray emission energies.
1. Will an incoming (gamma) photon of energy a bit more than the total required to excite all the shell electrons always get to cause fluorescence at all the wavelengths corresponding? Is there some probability that only one or two might glow?
2. For an incoming photon of (say) enough energy to excite all the electrons twice over, what happens to the excess energy? Can it ever go on to excite electrons in another atom?
3. Is there any forced order in the probabilities of electrons shells accepting energy? For example, do the L-shell electrons get raised by their quanta first, such that a photon with insufficient energy to affect the K-shell candidates, will still get glows from L-shell probable action? Is it take-up in order of energy, lowest first?
4. Is there some probability that a 20keV photon might excite a single Kβ1 ray from molybdenum at 17.48keV, without any others happening?
5.. Are there probabilities that an incoming photon, of enough energy to do something, might fail to do anything at all?
6.. Once a photon has caused some electron excitations, is it then "used up"? Does the excess then simply raise the temperature of the atom?
Please forgive the very basic nature of these questions. I do understand they may be inappropriate in this forum, but they do also fall outside the range of most other electronics design groups.
Last edited: