Dan Haronian
- 3
- 0
Hi
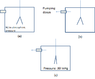
I am running the electroscope experiment in vacuum. This is shown in (a) in the uploaded figure . Once the aluminum leafs are charged the chamber is pump down (b). As soon the pressure decrease low enough (around relative pressure of 30 inHg) the leafs collapse to each other abruptly.
Here is a short video of the collapsing leafs:
).
There is still a gap between the two leafs but it's much less than in atmospheric pressure. This effect takes place every time I run the experiment.
In another experiment, the chamber is pumped down before the leafs are charged. The gap between the leafs is much smaller than that in atmospheric pressure.
Can anyone explain why?
I am running the electroscope experiment in vacuum. This is shown in (a) in the uploaded figure . Once the aluminum leafs are charged the chamber is pump down (b). As soon the pressure decrease low enough (around relative pressure of 30 inHg) the leafs collapse to each other abruptly.
Here is a short video of the collapsing leafs:
).
There is still a gap between the two leafs but it's much less than in atmospheric pressure. This effect takes place every time I run the experiment.
In another experiment, the chamber is pumped down before the leafs are charged. The gap between the leafs is much smaller than that in atmospheric pressure.
Can anyone explain why?
Attachments
Last edited: