Bolter
- 262
- 31
- Homework Statement
- Working out an estimation of molecule's diameter
- Relevant Equations
- V = 4/3*pi*r^3
Can anyone help me in what assumptions I can make and how to justify them for when working out diameter of the molecule in part b) of this question? You can see the method that I have used in my workings but how would I explain it in words?
I obtained the diameter to be 0.13 nano metres which seems like a sensible length for a molecules diameter?

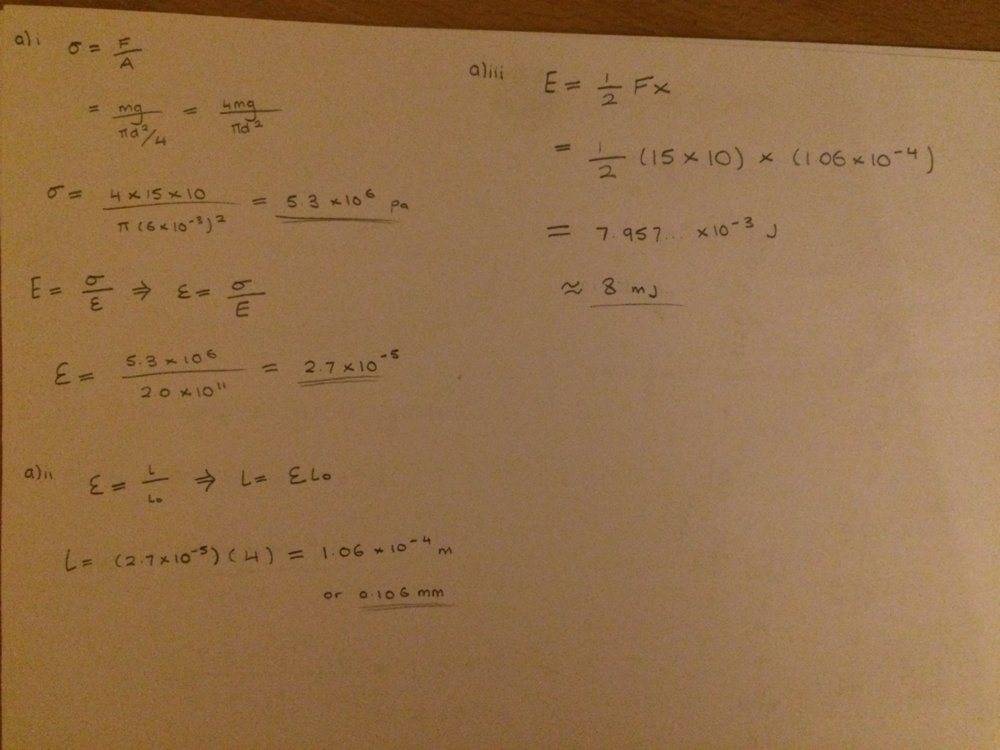
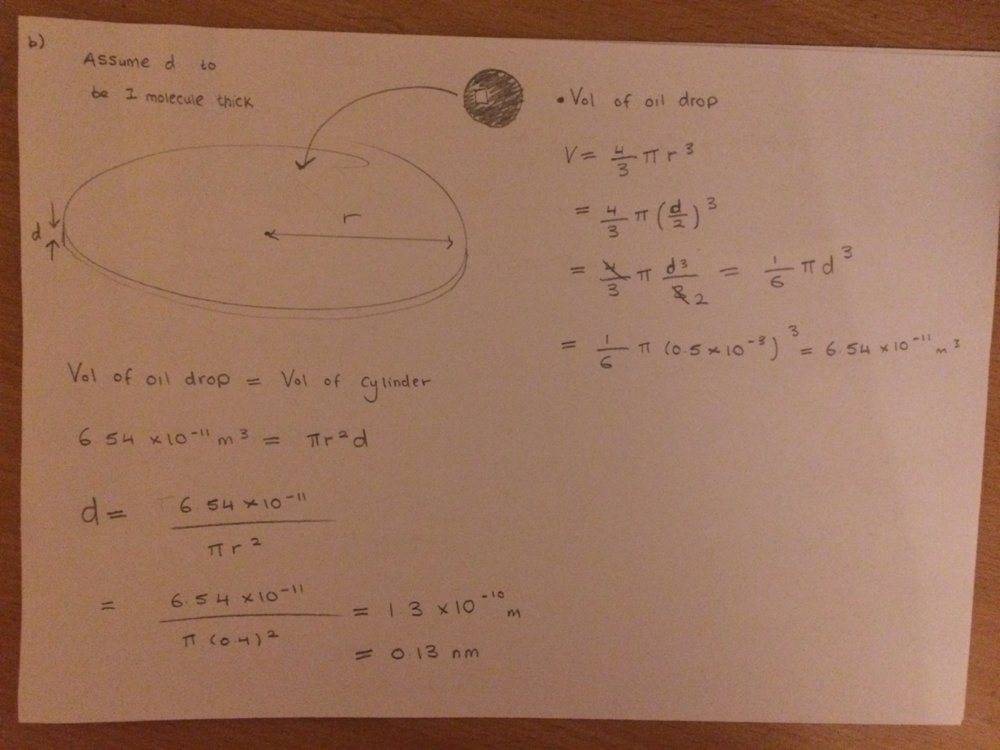
Thanks for any help! :)
I obtained the diameter to be 0.13 nano metres which seems like a sensible length for a molecules diameter?
Thanks for any help! :)