unscientific
- 1,728
- 13
Taken from Physics of Quantum Mechanics, by James Binney.
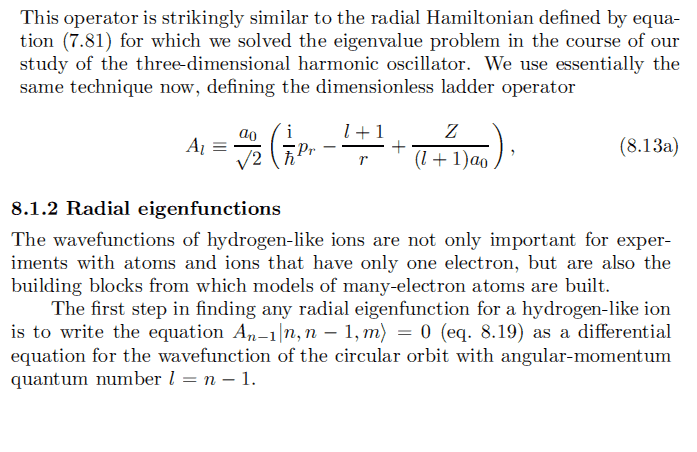
I try to calculate ##u_{n}^{l=n-2}##, something goes wrong:
Starting, we define operator A by:
A_{n-2} = \frac{a_0}{\sqrt 2}\left(\frac{i}{\hbar}p_r + \frac{1-n}{r} + \frac{Z}{(n-1)a_0}\right)
Substituting ##p_r = -i\hbar (\frac{\partial}{\partial r} + \frac{1}{r})##:
A_{n-2} = \frac{a_0}{\sqrt 2}\left( \frac{\partial}{\partial r} + \frac{2-n}{r} + \frac{Z}{(n-1)a_0}\right)
Thus, we want to solve:
\left(\frac{\partial}{\partial r} + \frac{2-n}{r} + \frac{Z}{(n-1)a_0} \right) u_{n}^{l=n-2} = 0
Solving by integrating factor method, we obtain:
u_n^{l=n-2} = A r^{n-2} e^{\frac{Z}{(n-1)a_0}r}
Normalizing,
u_n^{l=n-2} = \frac{1}{\sqrt{[2(n-1)]!}} \left(\frac{2Z}{(n-1)a_0}\right)^{\frac{3}{2}} \left(\frac{2Z}{(n-1)a_0}\right)^{n-2} e^{-\frac{Z}{(n-1)a_0}r}
This is similar to ##u_n^{l=n-1}##, simply replace n by n-1:
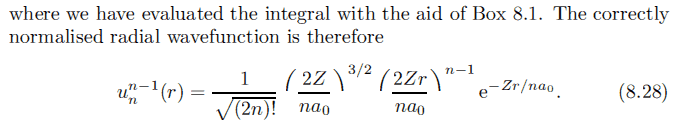
But when I substitute n = 2, so l = 0, I get ##u_2^0 = \frac{1}{\sqrt 2} \left(\frac{2Z}{a_0}\right)^{\frac{3}{2}} e^{-\frac{Z}{a_0}r}##
I get a completely different result from the book:
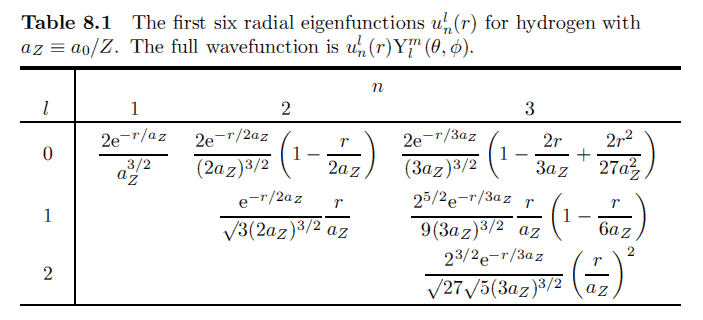
I'm not sure what's wrong with my derivation?
I try to calculate ##u_{n}^{l=n-2}##, something goes wrong:
Starting, we define operator A by:
A_{n-2} = \frac{a_0}{\sqrt 2}\left(\frac{i}{\hbar}p_r + \frac{1-n}{r} + \frac{Z}{(n-1)a_0}\right)
Substituting ##p_r = -i\hbar (\frac{\partial}{\partial r} + \frac{1}{r})##:
A_{n-2} = \frac{a_0}{\sqrt 2}\left( \frac{\partial}{\partial r} + \frac{2-n}{r} + \frac{Z}{(n-1)a_0}\right)
Thus, we want to solve:
\left(\frac{\partial}{\partial r} + \frac{2-n}{r} + \frac{Z}{(n-1)a_0} \right) u_{n}^{l=n-2} = 0
Solving by integrating factor method, we obtain:
u_n^{l=n-2} = A r^{n-2} e^{\frac{Z}{(n-1)a_0}r}
Normalizing,
u_n^{l=n-2} = \frac{1}{\sqrt{[2(n-1)]!}} \left(\frac{2Z}{(n-1)a_0}\right)^{\frac{3}{2}} \left(\frac{2Z}{(n-1)a_0}\right)^{n-2} e^{-\frac{Z}{(n-1)a_0}r}
This is similar to ##u_n^{l=n-1}##, simply replace n by n-1:
But when I substitute n = 2, so l = 0, I get ##u_2^0 = \frac{1}{\sqrt 2} \left(\frac{2Z}{a_0}\right)^{\frac{3}{2}} e^{-\frac{Z}{a_0}r}##
I get a completely different result from the book:
I'm not sure what's wrong with my derivation?