- #1
cardinal72491
- 2
- 0
I need help understanding the relationship between the excitation and emission spectra for a fluorescent material. This is not homework.
Let's say we have the following graph which plots the two spectra for a given material:
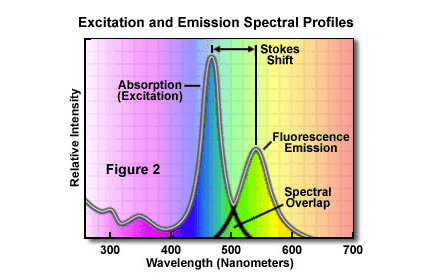
Two curves are shown - the excitation spectra and the emission spectra as well as the stokes shift (the difference in nanometers between the peak amplitudes of both curves).
From looking at the graph, the peak amplitude of the excitation spectrum is around 450, while the peak for the emission spectrum is around 550, making the stokes shift roughly 100 nm.
So my question is this: using this information how would I calculate the the emission wavelength given a particular light source. Below is my interpretation of what to do:
Step 1)
Let's say we have a monochromatic light at 400 nm with a spectral intensity value of 100. I would first look at the graph for the excitation spectra and see what the absorption value is for 400 nm. Let's call this value "A" (in the graph above the y-axis is not plotted so let's say its 0.1 for the sake of this example). I would then multiply light intensity * A (100 *0.1) and get an intensity of 10.
Step 2)
Next, to find the corresponding emission wavelength, I shift up the incoming wavelength by stokes shift. So stokes shift in this case is around 100 so we get 400 + 100 = 500 nm for the emitted light.
Step 3)
To find the intensity of the emitted light, I look at the emission spectrum graph and find the value for 500 nm (since the Y axis is unplotted let's say its value is 0.25). I would then modulate the absorbed light intensity by this outgoing value (10 * 0.25) and get an intensity of 2.5.
So based on my understanding a 400nm beam of light with a spectral intensity of 100 would cause this particular material to emit a beam of light at 500 nm at an intensity of 2.5.
Is the above a correct interpretation? The biggest thing I am unsure about here is whether stokes shift uniformly shifts over all wavelengths in the excitation spectra or if it is used only as a reference for measuring difference between peak amplitudes.
Let's say we have the following graph which plots the two spectra for a given material:
Two curves are shown - the excitation spectra and the emission spectra as well as the stokes shift (the difference in nanometers between the peak amplitudes of both curves).
From looking at the graph, the peak amplitude of the excitation spectrum is around 450, while the peak for the emission spectrum is around 550, making the stokes shift roughly 100 nm.
So my question is this: using this information how would I calculate the the emission wavelength given a particular light source. Below is my interpretation of what to do:
Step 1)
Let's say we have a monochromatic light at 400 nm with a spectral intensity value of 100. I would first look at the graph for the excitation spectra and see what the absorption value is for 400 nm. Let's call this value "A" (in the graph above the y-axis is not plotted so let's say its 0.1 for the sake of this example). I would then multiply light intensity * A (100 *0.1) and get an intensity of 10.
Step 2)
Next, to find the corresponding emission wavelength, I shift up the incoming wavelength by stokes shift. So stokes shift in this case is around 100 so we get 400 + 100 = 500 nm for the emitted light.
Step 3)
To find the intensity of the emitted light, I look at the emission spectrum graph and find the value for 500 nm (since the Y axis is unplotted let's say its value is 0.25). I would then modulate the absorbed light intensity by this outgoing value (10 * 0.25) and get an intensity of 2.5.
So based on my understanding a 400nm beam of light with a spectral intensity of 100 would cause this particular material to emit a beam of light at 500 nm at an intensity of 2.5.
Is the above a correct interpretation? The biggest thing I am unsure about here is whether stokes shift uniformly shifts over all wavelengths in the excitation spectra or if it is used only as a reference for measuring difference between peak amplitudes.