- #1
sukharef
- 54
- 0
Hey!
In https://www.researchgate.net/publication/230937856_Optical_properties_of_graphene article (Optical properties of graphene) I found out the frequency is expressed in Kelvins.
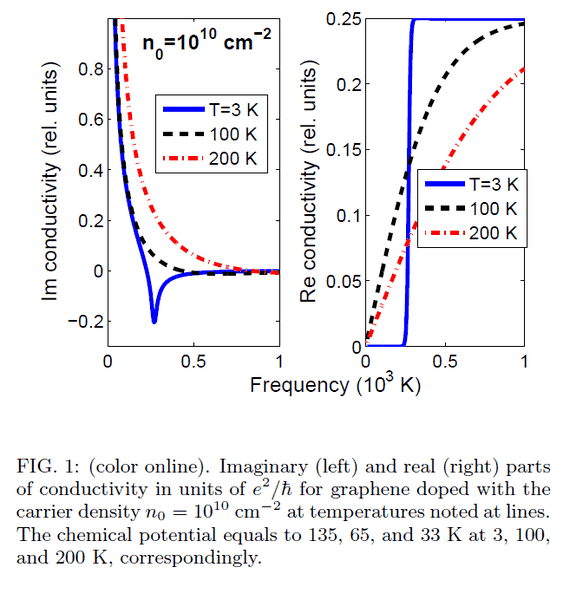
Could you help me with it - how did the author do this unit transformation?
Thanks in advance!
In https://www.researchgate.net/publication/230937856_Optical_properties_of_graphene article (Optical properties of graphene) I found out the frequency is expressed in Kelvins.
Could you help me with it - how did the author do this unit transformation?
Thanks in advance!
Last edited by a moderator: