Govind
- 11
- 1
Let's consider a reaction A (reactant) -> B(product) and activated complex is denoted by C.
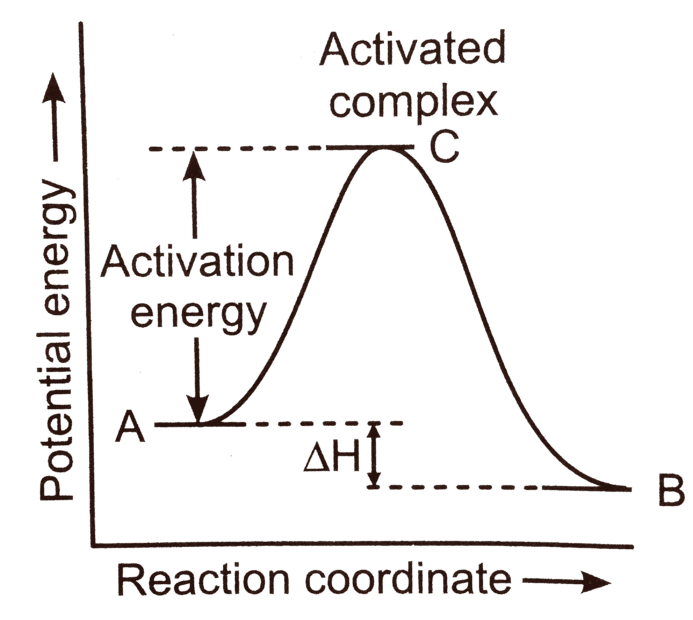
This graph ( potential energy vs reaction coordinate ) tells us that reactant need some amount of activation energy (Ea) to convert in product, which has low potential energy which is shown here in terms of enthalpy ∆H. We can assume from this graph that activation represent same kind of potential energy between A (reactant) and C (activated complex ) that Enthalpy ∆H represent between A and B (product).
Now look at another graph of reaction (Gibbs free energy vs extent of reaction)
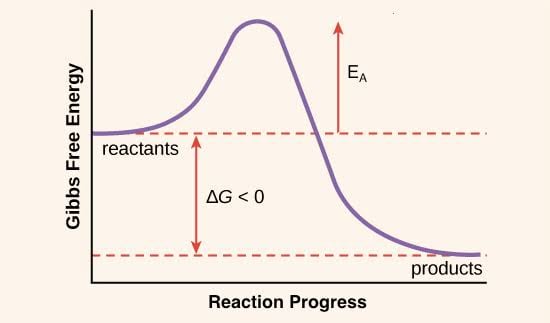
This graph represents that activation energy is difference between Gibbs free energy of reactant and activated complex or there is also possibility that the activation energy shown here is not arrhenius activation energy Ea but it is Gibbs energy of activation ΔG‡ according to transition state theory.
Q. But to perform a reaction what amount of energy we need to supply to reactants arrhenius activation energy Ea or gibbs free energy of activation ΔG‡ ? I think it's ΔG‡ as defination of Gibbs free energy states - minimum amount of work needed to supply for a non spontaneous reaction (here A -> C ) to be happened but then why arrhenius theory states that - for reactants to transform into products, they must first acquire a minimum amount of energy, called the activation energy Ea ?
And also what these two energies represent physically in terms of bonds , interatomic interactions etc ?
Mathematical equations -
ΔG‡ = ∆H‡ - T∆S‡
ΔG‡ = Ea - RT - T∆S‡ ( ∆H‡ = Ea - RT )
This graph ( potential energy vs reaction coordinate ) tells us that reactant need some amount of activation energy (Ea) to convert in product, which has low potential energy which is shown here in terms of enthalpy ∆H. We can assume from this graph that activation represent same kind of potential energy between A (reactant) and C (activated complex ) that Enthalpy ∆H represent between A and B (product).
Now look at another graph of reaction (Gibbs free energy vs extent of reaction)
This graph represents that activation energy is difference between Gibbs free energy of reactant and activated complex or there is also possibility that the activation energy shown here is not arrhenius activation energy Ea but it is Gibbs energy of activation ΔG‡ according to transition state theory.
Q. But to perform a reaction what amount of energy we need to supply to reactants arrhenius activation energy Ea or gibbs free energy of activation ΔG‡ ? I think it's ΔG‡ as defination of Gibbs free energy states - minimum amount of work needed to supply for a non spontaneous reaction (here A -> C ) to be happened but then why arrhenius theory states that - for reactants to transform into products, they must first acquire a minimum amount of energy, called the activation energy Ea ?
And also what these two energies represent physically in terms of bonds , interatomic interactions etc ?
Mathematical equations -
ΔG‡ = ∆H‡ - T∆S‡
ΔG‡ = Ea - RT - T∆S‡ ( ∆H‡ = Ea - RT )