AliSkully
- 3
- 0
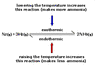
Can someone explain why when you increase the temperature on an endothermic reaction, it yields more products? Is it because the activation energy is higher in an endothermic reaction, so there is more time for the products to form? also why does decreasing the temperature yield more products in an exothermic reaction. I'm having trouble grasping this.
cheers
cheers