- 6,735
- 2,434
Homework Statement
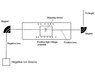
I have a source producing ions of He: He2+ and He+, they are accelerated towards a tandem accelerator, se atteched figure. They are accelerated with a voltage towards the first magnet, were a selection of the ions are made. We only want the He2+ ions the proceed to the tandem accelerator.
Show how this is done in the first analysis magnet.
answer according to my teacher:
the He2+ will get higher energy and therefore less bent, larger radius, then the He+ ions.
Homework Equations
andThe Attempt at a Solution
But according to this, I found that is the opposite, becase F= qvB and the bigger charge, the more force, and hence smaller radius.
|F| = qvB \Rightarrow r = \frac{mv}{qB}
m and B is the (practically) same for all, I have He+ and He2+ ions. T is kinetical energy.
|F| = qvB \Rightarrow r = \frac{mv}{qB}r\propto \frac{v}{q}U=T=qV = mc^{2} \Rightarrow v=c\sqrt{1-\left( \frac{mc^{2}}{qV+mc^{2}}\right) ^{2}}}<br />Ratio for radius of He2+ and He+ "r(2q)/r(q)"
gives me that He2+ has smaller radius than He+ if they are accelerated with the same potential V and is bent i same magnetiv field.
Non-relativistic gives me:
r\propto \frac{v}{q} v=\sqrt{\frac{2qV}{m}} r\propto \frac{\sqrt{\frac{2qV}{m}}}{q} = \frac{constant}{\sqrt{q}}
LOL help =)
Last edited: