ecneicS
- 64
- 0
I was watching my friends smoke hookah last night thinking about how it works. I drew this simplified figure to help for anyone who doesn't know what a bong or hookah looks like. I know that it works on the principle of suction, but what IS suction?
1. Why do different results occur when I suck on the top with my lips fully around the opening (insert sex joke here) aka creating a suction, compared to when I just suck in air with my mouth very close to the opening?
2. Why does sucking air from the taller tube cause air from the smaller tube to enter the the water?
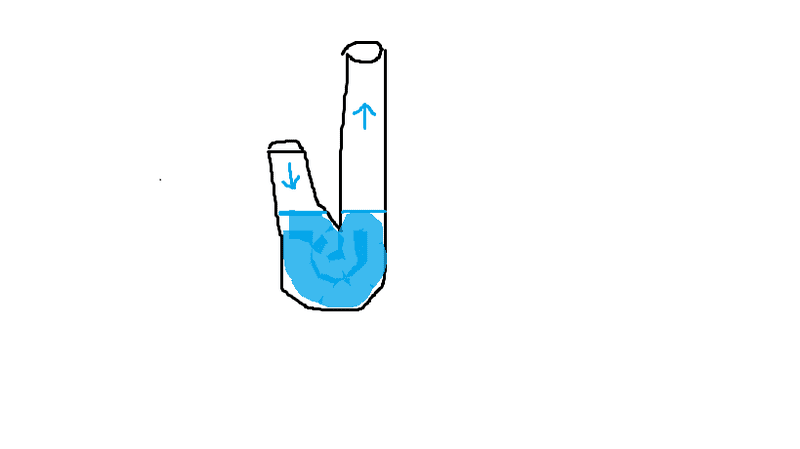
1. Why do different results occur when I suck on the top with my lips fully around the opening (insert sex joke here) aka creating a suction, compared to when I just suck in air with my mouth very close to the opening?
2. Why does sucking air from the taller tube cause air from the smaller tube to enter the the water?