omri3012
- 60
- 0
infinite potential well and the uncertainty principle
the solution for Schroedinger equation in infinite potential well satisfy the following
energy levels:
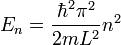
where l is the width of the well.
E can't be zero since then \psi=0 so there isn't any particle in the well . i read in
a book that "there is a tight connection between this fact (E\neq0) and the
uncertainty principle", what exactly is the connection?
the solution for Schroedinger equation in infinite potential well satisfy the following
energy levels:
where l is the width of the well.
E can't be zero since then \psi=0 so there isn't any particle in the well . i read in
a book that "there is a tight connection between this fact (E\neq0) and the
uncertainty principle", what exactly is the connection?
Last edited: