Oldor
- 6
- 0
Homework Statement
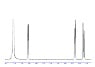
How many peaks will there be in the NMR spectrum of Malic Acid, HOOC CH2 CH(OH) COOH, WITHOUT D2O as the solvent (i.e. Hydrogens in Hydroxyl groups WILL have corresponding peaks)?
The Attempt at a Solution
I thought the two -COOH groups will be identical Hydrogen environments, so there would only be one peak for both. There will be one environment with the single H bonded to the Carbon that is also bonded to the OH; one environment with the single H in the -OH bonded to that same Carbon; and one environment with the two H's bonded to the remaining Carbon. A total of 4 peaks without D2O (or 5 if the Carboxylic acid groups are not identical)?