SweatingBear
- 119
- 0
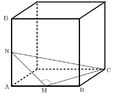
I received an excellent question about significant figures when there is no actual numerical data given. In the figure below, we have a cube circumscribing a triangle and the task is to find the angle at M. N and M are midpoints of the cube's side.
View attachment 1016
By using the Pythagorean theorem a few times and ultimately law of cosines, one can arrive at
$$\cos (\text{M}) = \frac { \frac 94 - \frac 12 - \frac 54 }{ -\sqrt{\frac 52} } \, ,$$
where one solution is $$M \approx 108.4349488^\circ$$. Here is the problem: How many significant figures ought one to have for the angle? We were not given any numerical data, so what ought one to do? Integral values seems to be a natural, conventional practice; however, in terms of significant figures, the questions becomes much more interesting.
View attachment 1016
By using the Pythagorean theorem a few times and ultimately law of cosines, one can arrive at
$$\cos (\text{M}) = \frac { \frac 94 - \frac 12 - \frac 54 }{ -\sqrt{\frac 52} } \, ,$$
where one solution is $$M \approx 108.4349488^\circ$$. Here is the problem: How many significant figures ought one to have for the angle? We were not given any numerical data, so what ought one to do? Integral values seems to be a natural, conventional practice; however, in terms of significant figures, the questions becomes much more interesting.
Attachments
Last edited: