mycotheology
- 86
- 0
Heres the chiral center:
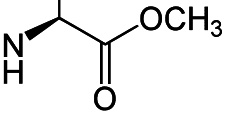
I want to change the chirality of the amino group. I was wondering if I can do it through some kind of nucleuphilic addition to the carbonyl group, while adding something to adding a metal that will coordinate the amino group and drag it back or force it to change chirality or something like that.
Another idea I had was to use some kind of SN2 to invert the stereochemistry, but I have no idea how to do that. Is there any way I can remove a hydrogen from that chiral center in order to temporarily turn it trigonal planar, then when I re-hydrogenate it, at least I'd have a racemate.
Or would it be possible to tenporarily make the amino group achiral by converting it to a double bond or something? Then when its trigonal planar, I suppose I could figure out how to get the right stereochemistry.
Or here's another idea, maybe I could deprotect the carboxyl group, then run a kolbe electrolysis on it, but I will put an excess of mono esterified oxalic acid so that the majority of the my product will dimerise with the oxalic acid, rather than itself. Only problem there, is the oxalic acid will dimerise with itself, but I suppose I could add tiny amounts of the oxalic acid in at a time. This seems like it just might work.
Any ideas?
I want to change the chirality of the amino group. I was wondering if I can do it through some kind of nucleuphilic addition to the carbonyl group, while adding something to adding a metal that will coordinate the amino group and drag it back or force it to change chirality or something like that.
Another idea I had was to use some kind of SN2 to invert the stereochemistry, but I have no idea how to do that. Is there any way I can remove a hydrogen from that chiral center in order to temporarily turn it trigonal planar, then when I re-hydrogenate it, at least I'd have a racemate.
Or would it be possible to tenporarily make the amino group achiral by converting it to a double bond or something? Then when its trigonal planar, I suppose I could figure out how to get the right stereochemistry.
Or here's another idea, maybe I could deprotect the carboxyl group, then run a kolbe electrolysis on it, but I will put an excess of mono esterified oxalic acid so that the majority of the my product will dimerise with the oxalic acid, rather than itself. Only problem there, is the oxalic acid will dimerise with itself, but I suppose I could add tiny amounts of the oxalic acid in at a time. This seems like it just might work.
Any ideas?
Last edited: