Karfen
- 1
- 0
- Homework Statement
- Estimate the molar specific heat of the air at constant volume Cv by taking into account
major molecules in the air. Plot it as a function of absolute temperature and discuss its
significance.
- Relevant Equations
- The major chemical elements of air are nitrogen(78%) and oxygen(22%).
The molar specific heat of the air at constant volume for diatomic molecule:
Cv=3/2R (Low Temperature)
Cv=5/2R(High Temperature)
Cv=7/2R(Higher Temperature)
And this is the Cv graph for hydrogen:
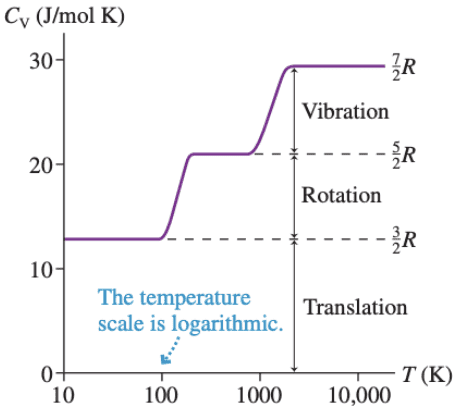
I think the Cv graph for air is similar with this graph. But I don't know the answer, can someone tell me?
I think the Cv graph for air is similar with this graph. But I don't know the answer, can someone tell me?