SUMMARY
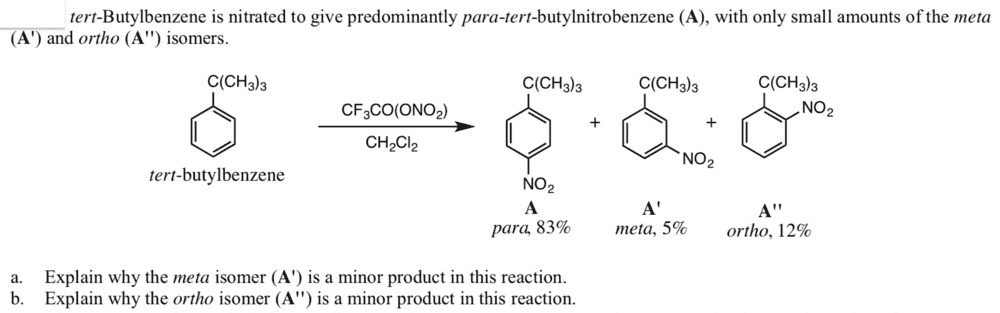
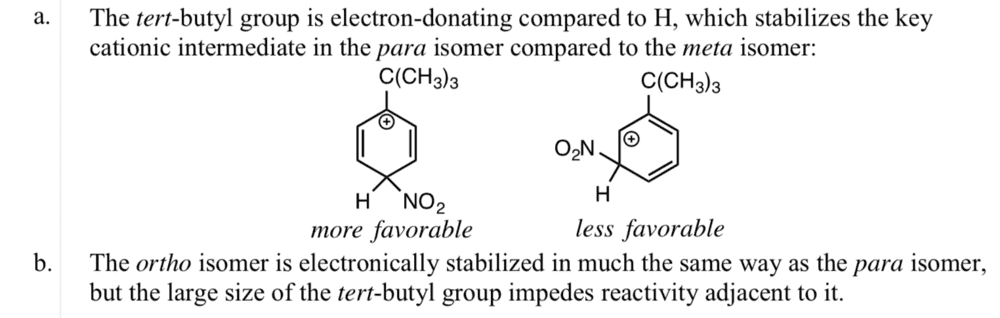
The discussion centers on the isomers of tert-butylnitrobenzene, specifically the resonance structures of para and meta isomers and the stabilization of the ortho isomer. The nitration mechanism of benzene is highlighted, particularly the generation of the nitronium ion (NO2+) using a non-classical nitrating agent, trifluoroacetic acid, in a non-aqueous solution like methylene dichloride. The classical method involving a sulfuric/nitric acid mixture is contrasted with this approach, emphasizing the need for further investigation into the advantages of the alternative nitrating agent.
PREREQUISITES
- Understanding of electrophilic aromatic substitution mechanisms
- Familiarity with resonance structures in organic chemistry
- Knowledge of nitration agents and their properties
- Basic concepts of non-aqueous reaction environments
NEXT STEPS
- Research the mechanism of electrophilic nitration of benzene using sulfuric/nitric acid mixtures
- Explore the properties and applications of trifluoroacetic acid as a nitrating agent
- Study the resonance structures of nitro-substituted benzene derivatives
- Investigate the effects of solvent choice on reaction mechanisms in organic chemistry
USEFUL FOR
Chemistry students, organic chemists, and researchers interested in electrophilic aromatic substitution and the synthesis of nitro compounds.