SUMMARY
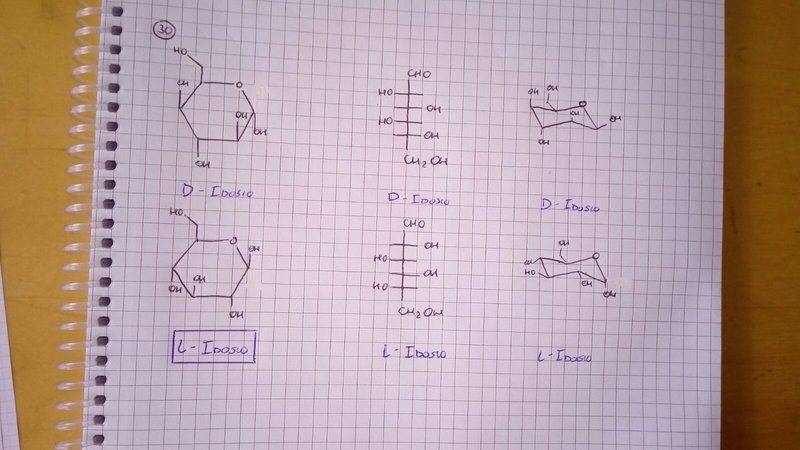
The discussion focuses on the structural representation of L-Idose using Haworth notation, addressing its chirality and stereocenters. Participants clarify that L-Idose is indeed chiral, with specific stereocenters identified at C2, C3, C4, and C5. The functional groups present in L-Idose include hydroxyl groups and a carbonyl group. The confusion regarding the correct representation of L-Idose is resolved by referring to established resources on D- and L-sugars.
PREREQUISITES
- Understanding of Haworth projection for cyclic sugars
- Knowledge of chirality and stereocenters in organic chemistry
- Familiarity with functional groups in carbohydrates
- Basic concepts of D- and L-sugar nomenclature
NEXT STEPS
- Study the Haworth projection method for other monosaccharides
- Learn about the stereochemistry of carbohydrates
- Explore the differences between D- and L-sugars in detail
- Investigate the role of functional groups in sugar reactivity
USEFUL FOR
Chemistry students, organic chemists, and anyone studying carbohydrate structures and their stereochemistry.