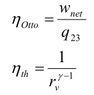physea
- 211
- 3
Hello guys,
I am a bit confused. Is it that PV/T=constant for ALL the processes of ALL cycles, like Otto, Diesel, Rankine?
I know that PV=mRT, so does that mean that PV/T=constant all the time?
Secondly, I am a bit confused as I read that PV^γ=constant. What is that exactly? Where does it come from and when does it apply?
Thanks!
I am a bit confused. Is it that PV/T=constant for ALL the processes of ALL cycles, like Otto, Diesel, Rankine?
I know that PV=mRT, so does that mean that PV/T=constant all the time?
Secondly, I am a bit confused as I read that PV^γ=constant. What is that exactly? Where does it come from and when does it apply?
Thanks!