Avardia
- 6
- 0
So I've been looking at covalent bonds and come across the approx you can do of the molecular orbital for ##H^+_2## by just summing two 1s orbitals, the method is called the linear combinations of atomic orbitals, and you get what is below which I believe is exact in this case since the 1s orbital is all there is. And the orbital is just the wavefunction of electron.
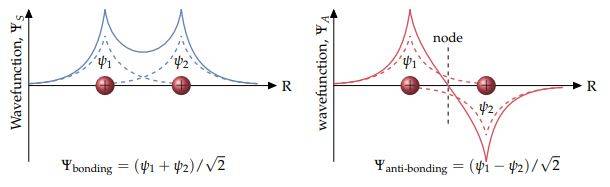
So one is the bonding and the other is anti-bonding orbital. My question is why in the anti-bonding orbital do we have one of the summed 1s wavefunctions as negative?
So one is the bonding and the other is anti-bonding orbital. My question is why in the anti-bonding orbital do we have one of the summed 1s wavefunctions as negative?