nishantve1
- 74
- 1
Consider a complex with Central Metal atom M
A and B are monodentate ligands .
Consider a compound with formula as
[M A4 B2] . Textbook and the web says there can be only 2 possible isomers of this compound .
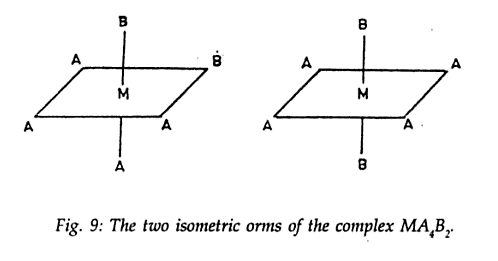
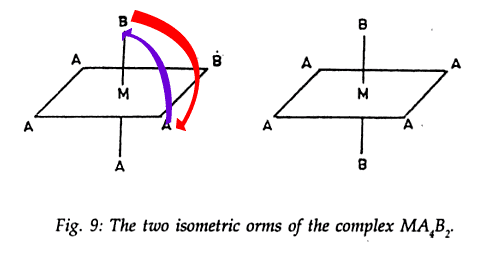
What I say is, why can't I in the first image put A on the top and bring B towards the bottom something like this
 .
.
And them form these,
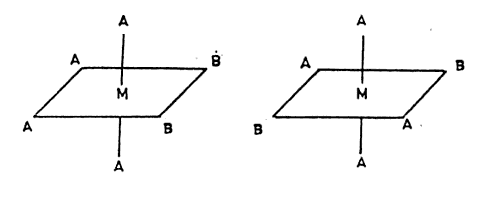
That will give a total of 2 new ones I formed and the two old ones, 4 isomers .
Why is this not possible, I mean why can't a occupy the two places ?
A and B are monodentate ligands .
Consider a compound with formula as
[M A4 B2] . Textbook and the web says there can be only 2 possible isomers of this compound .
What I say is, why can't I in the first image put A on the top and bring B towards the bottom something like this
And them form these,
That will give a total of 2 new ones I formed and the two old ones, 4 isomers .
Why is this not possible, I mean why can't a occupy the two places ?
Last edited: