Astronuc said:
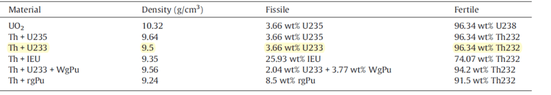
The 3.66% U235 or U233 would imply that the remainder of the metal (Th) is 96.34%. Note the densities to the mixed-oxide and are less than the theoretical density (due to porosity in the ceramic material) and vary slightly due to the differences in mass of the fissile nuclides.
Of course, I'm referring to (U,Th)O
2.
Aly_19f said:
But by applying the same method in the MOX it is always >1.
Probably the method assumes a common density, which is a reasonable assumption for UO
2, but not MOX, but of course, the atom fractions should add to one.
Aly_19f said:
Is there any textbook or tutorial about burnup calculations?
I would think most textbooks for Introductory Nuclear Engineering or Nuclear Reactor Theory would have some background or examples. This is essentially a basic chemistry problem.
The atom density, N, is given by N = ρ Av / M, where ρ is the density, Av is Avogadro's number and M is the molecular mass.
When calculating atomic densities of mixed oxides, MO
2, one may apply the rule of mixtures, but must understand the relationship of mass, density and volume, and it can get complicated when each heavy metal element has multiple isotopes, e.g.,
233U,
235U,
238U, and there may be
234U and
236U, and with Pu,
238Pu,
239Pu,
240Pu,
241Pu and
242Pu.
Rule of mixtures -
https://en.wikipedia.org/wiki/Rule_of_mixtures
https://www.sciencedirect.com/topics/engineering/rule-of-mixture-equation
With mixed oxides, one has to remember that there is one M (Th,U,Pu) for each O
2.
So if we want to calculate atomic densities of M, then we need to keep in mind that we would first calculate the molecular density of MO
2 from the mass density, and then multiply by the mass ratio M/MO
2.
Also, one must remember the physical density is less than the theoretical density, because ceramics do not sinter to 100% of theoretical density. There is some inherent porosity, which is process specific.
The theoretical densities of the compounds interest are:
ThO
2, 10.00 g/cm
3
UO
2, 10.96 g/cm
3, although some use 10.97 or 10.98, which might indicate an influence of the proportions of
235U and
238U in the UO
2; getting a pure amount of
235U or
238U is exceedingly difficult, or there could be some porosity or other impurity.
PuO
2, 11.46 g/cm
3
One should note the ratio of M to MO
2. For example, for UO
2, one could approximate it by 238/(238+2*16) = 238/270 = 0.8815. If one wishes to be more precise, then one would have to account for the proportions of
235U and
238U. For example, for 5% enrichment, the ratio is (0.05*235+0.95*238)/(0.05*235+0.95*238+2*16) = (237.85)/(237.85+32) = 0.8814. So, it's a small difference, since the atomic masses of the isotopes are very close.
For densities of mixtures of oxides, one can use the atom% of the metals, which are close to the wt%. For example, the theoretical density of (U
0.8, Pu
0.2)O
2 is approximately 11.08 g/cm
3. Using the densities above for UO
2 = 10.96 and PuO
2 = 11.46, then multiplying by the atomic fractions 0.8 and 0.2, respectively, one obtains 10.96 g/cm
3 * 0.8 + 11.46 g/cm
3 * 0.2 = 11.06 g/cm
3.
In the above example, given the 3.66%
233U enrichment in (U,Th)O
2, and densities of 10.96 g/cm
3 and 10 g/cm
3 for UO
2 and ThO
2, respectively, then the theoretical density of the mixture/compound is 0.0366*10.96 g/cm
3 + 0.9634 * 10 g/cm
3 = 10.04 g/cm
3. So, the theoretical density (TD) is slighly greater than that of ThO
2. The table one provided has the density at 9.5 g/cm
3, from which one would infer that the mixed oxide ceramic has a density of ~0.95 of theoretical, which is a typical target of oxide ceramic nuclear fuel. In the distant past, ceramic densities were lower, ~0.87-0.94 TD.