Discussion Overview
The discussion centers on the non-linear conductivity of tap water as observed through experimental measurements involving stainless steel nails immersed in the water. Participants explore the implications of their findings, the potential for experimental errors, and the underlying physical principles that may explain the observed behavior.
Discussion Character
- Exploratory
- Technical explanation
- Debate/contested
- Experimental/applied
Main Points Raised
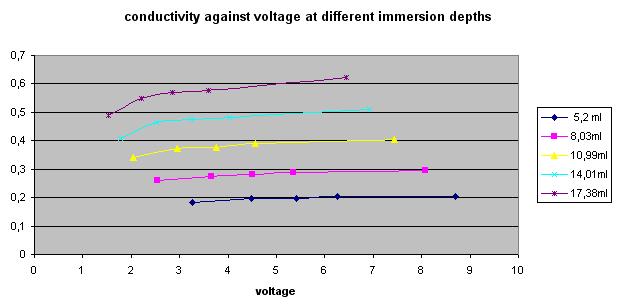
- One participant notes that conductivity between nails in tap water decreases at low voltages, suggesting a deviation from expected linear behavior.
- Another participant proposes that a "contact potential" phenomenon might explain the low current at lower voltages, along with a possible phase term related to charge travel time.
- A participant asserts that water is not a linear ohmic medium, indicating a fundamental property of water's conductivity.
- One participant identifies experimental errors related to the AC supply voltage dropping under load and temperature variations, leading to imprecise measurements.
- Another participant reflects on the observation that tap water seems to follow Ohm's law closely at certain voltage ranges, questioning the conditions under which this holds true.
- Further discussion raises the challenge of understanding why tap water exhibits linear conductivity under specific conditions but may not do so under others, such as varying temperatures or concentrations.
- One participant expresses uncertainty about calculating depth based on resistance due to potential non-linear behavior and discusses the effects of heating on conductivity.
- Another participant shares plans to improve experimental precision by using a constant temperature water source and a thermocouple for accurate temperature measurements.
- Concerns are raised about the lack of information available online regarding the non-linearity of electrolytes, despite extensive discussions on temperature-related non-linearity.
Areas of Agreement / Disagreement
Participants express a mix of agreement and disagreement regarding the nature of water's conductivity. While some acknowledge the potential for experimental error, others maintain that the observed non-linearity may be a genuine characteristic of tap water. The discussion remains unresolved with multiple competing views on the underlying causes of the observed phenomena.
Contextual Notes
Limitations include potential experimental errors, dependence on the specific conditions of the measurements (such as voltage and temperature), and the lack of established literature on the non-linearity of electrolytes.
Who May Find This Useful
This discussion may be of interest to those studying electrochemistry, experimental physics, or anyone exploring the properties of conductive materials, particularly in relation to water and electrolytes.