Discussion Overview
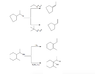
The discussion revolves around predicting the major product and identifying the type of reaction mechanism (E1/E2/SN1/SN2) in organic chemistry, specifically focusing on elimination reactions involving alkyl halides. Participants are reviewing and checking each other's proposed solutions and reasoning.
Discussion Character
- Homework-related
- Debate/contested
- Technical explanation
Main Points Raised
- One participant suggests that the major products from the reactions are E2, E1, SN1, and E1.
- Another participant agrees with the initial assessment but notes a permutation error in the last two solutions.
- A different participant proposes an alternative sequence of E2 (strong base), E2 (strong bulky base leading to Hoffman elimination), E1, and an acid-base reaction followed by SN1, while also mentioning a shift in the images.
- There is a question raised about the terminology of referring to E1/E2 eliminations as “Hoffman elimination.”
- One participant argues that the use of “Hoffman elimination” is appropriate when a bulky base like tert-butoxide is involved, as it leads to the formation of the less stable alkene, which is considered the Hoffman product.
- Another participant counters that the term “Hoffman elimination” is not standard for alkyl halide reactions with strong bases and emphasizes that it should only apply when the leaving group is a quaternary amine.
- Further clarification is provided that the term “Hoffman product” is being used, but it may not align with standard nomenclature.
Areas of Agreement / Disagreement
Participants express differing views on the appropriate terminology for elimination reactions and the classification of products, indicating a lack of consensus on the use of “Hoffman elimination” in this context.
Contextual Notes
There are unresolved issues regarding the definitions and nomenclature of elimination reactions, particularly concerning the conditions under which the term “Hoffman elimination” is applicable.