Lamdbaenergy
- 35
- 1
So I was just looking around today and stumbled upon something called Planck's law. I saw an equation and quite few more of them that looked like this,
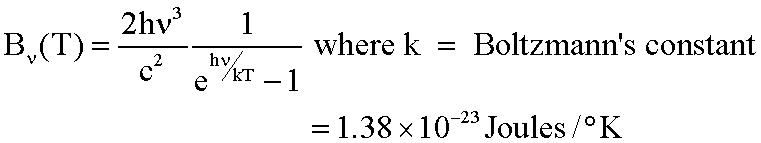
I'm familiar with all of the other variables and constants already, but don't get the E (hv/kT) part, yet. Is E the energy of the photons you are supposed to raise it to by the POWER of (hv/kT)? And what does the result you get from this whole equation tell you about the radiation coming out of the black body? Don't blind me with too much math if you don't really have to and thanks!
I'm familiar with all of the other variables and constants already, but don't get the E (hv/kT) part, yet. Is E the energy of the photons you are supposed to raise it to by the POWER of (hv/kT)? And what does the result you get from this whole equation tell you about the radiation coming out of the black body? Don't blind me with too much math if you don't really have to and thanks!