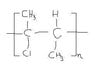
Polymerization of 2-Chloro-2-Butene: Solution
- Thread starter Macroer
- Start date
-
- Tags
- Polymer
Click For Summary
SUMMARY
The discussion focuses on the addition polymerization of 2-chloro-2-butene, detailing the initiation and termination processes involved in polymer formation. The initiator (I) reacts with 2-chloro-2-butene to form a radical, leading to the growth of the polymer chain. The end groups of the polymer are specified as (CH3)(I)C- and (CH3)(Cl)(I)C-, which are crucial for understanding the structure of the final polymer. Participants clarify the importance of accurately representing the end groups in the polymer structure.
PREREQUISITES- Understanding of addition polymerization mechanisms
- Familiarity with radical initiators in polymer chemistry
- Knowledge of organic chemistry nomenclature and structure representation
- Experience with drawing polymer structures and end groups
- Study the mechanism of addition polymerization in detail
- Learn about different types of radical initiators and their roles
- Explore the significance of end groups in polymer chemistry
- Investigate the properties and applications of polymers derived from 2-chloro-2-butene
Chemistry students, organic chemists, and professionals involved in polymer science who are looking to deepen their understanding of polymerization processes and the structural characteristics of polymers.
Similar threads
Chemistry
Elimination question on alkyl halide
- · Replies 0 ·
- · Replies 2 ·
- · Replies 1 ·
- · Replies 3 ·
- · Replies 2 ·
- · Replies 3 ·
- · Replies 11 ·