Callmelucky
- 144
- 30
- Homework Statement
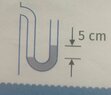
- to a container filled with gas, U shaped pipe is attached, as shown in the picture(picture below). What is a gas pressure in the container if the height of the pillar of mercury in barometer is 740 mm?
- Relevant Equations
- pressure = density * g * height
Can someone please tell me where I am wrong, here goes the question:
to a container filled with gas, U shaped pipe is attached, as shown in the picture(picture below). What is a gas pressure in the container if the height of the pillar of mercury in barometer is 740 mm?
The way I solved it is: pressure of mercury(740mm) + 5 cm difference in U pipe --> 0.740 m * 13600 * 9.81 + 0.05 * 13600 * 9.81 = 105398.64 Pa. But the answer at the and of the textbook is 100 062 Pa.
Thank you.
to a container filled with gas, U shaped pipe is attached, as shown in the picture(picture below). What is a gas pressure in the container if the height of the pillar of mercury in barometer is 740 mm?
The way I solved it is: pressure of mercury(740mm) + 5 cm difference in U pipe --> 0.740 m * 13600 * 9.81 + 0.05 * 13600 * 9.81 = 105398.64 Pa. But the answer at the and of the textbook is 100 062 Pa.
Thank you.