- #1
Bolter
- 262
- 31
- Homework Statement
- See image below
- Relevant Equations
- Rydberg equation
Here is a question I have been given

I have started by setting up the formula and rearranging for n_2
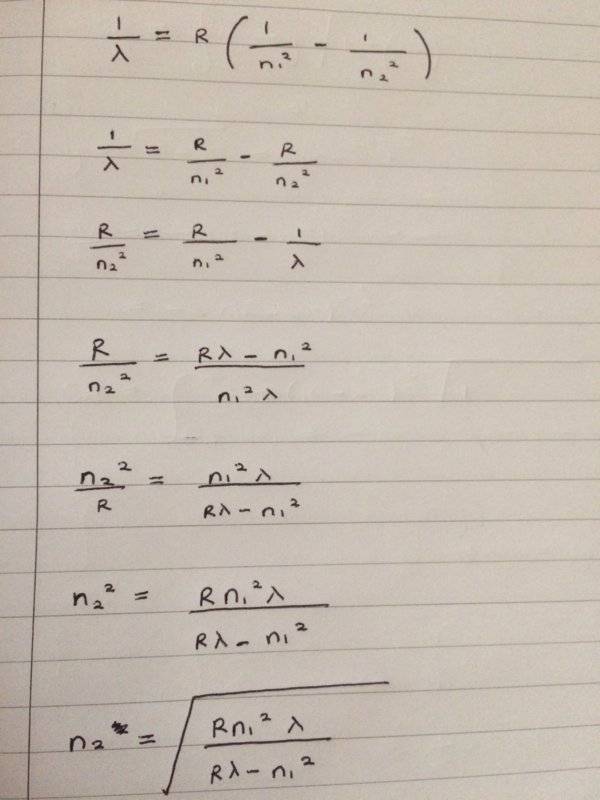
Only problem is that I do not know the quantum number for ground state? What value do I sub in for n_1?
Any help would be appreciated! Thanks
I have started by setting up the formula and rearranging for n_2
Only problem is that I do not know the quantum number for ground state? What value do I sub in for n_1?
Any help would be appreciated! Thanks