ohspyro89
- 13
- 0
I hope I can make this understandable.
I'm working on a design to utilize combustion of propane to cycle a piston. I'll attach a gif to make it more simple to understand. When the cylinder is filled with an ideal 4% of propane (green) it'll combust (turns yellow) to push back the cylinder.
The issue I'm having here is that I'm not sure how to use the combustion value of propane (2500 btu/ft3 or 93300 kJ/m3) to figure out how much force the piston will be pushed back.
As of now, the dimensions of the whole system aren't defined. I feel like I need to get a feel of how much force can be utilized from the propane combustion. It could be assumed that the combustion chamber is 2.5" long and 1" in diameter.
Also, there is an open port at the front to exhaust the combusted propane to propel a projectile. This port is 0.125'' in diameter.
The main objective is to have enough force from the propane combustion to cycle the bolt fully. The exhaust port, on top with the arrows, can be moved also to give the bolt more time to gain momentum.
I've been out of physics for a while now, and I've lost my touch for sure. This is a lot for me to try to compute, and I'd rather not start prototyping and find out that my dimensions and weights are incredibly far off.
Thanks a lot!
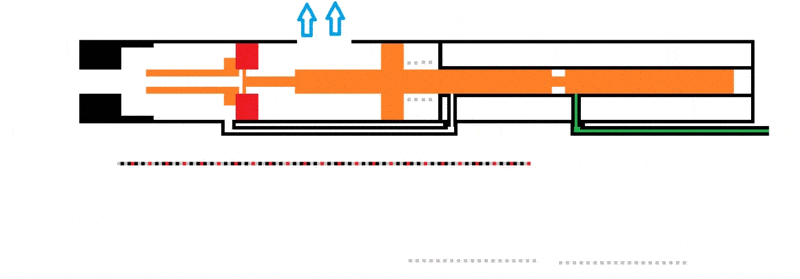
I'm working on a design to utilize combustion of propane to cycle a piston. I'll attach a gif to make it more simple to understand. When the cylinder is filled with an ideal 4% of propane (green) it'll combust (turns yellow) to push back the cylinder.
The issue I'm having here is that I'm not sure how to use the combustion value of propane (2500 btu/ft3 or 93300 kJ/m3) to figure out how much force the piston will be pushed back.
As of now, the dimensions of the whole system aren't defined. I feel like I need to get a feel of how much force can be utilized from the propane combustion. It could be assumed that the combustion chamber is 2.5" long and 1" in diameter.
Also, there is an open port at the front to exhaust the combusted propane to propel a projectile. This port is 0.125'' in diameter.
The main objective is to have enough force from the propane combustion to cycle the bolt fully. The exhaust port, on top with the arrows, can be moved also to give the bolt more time to gain momentum.
I've been out of physics for a while now, and I've lost my touch for sure. This is a lot for me to try to compute, and I'd rather not start prototyping and find out that my dimensions and weights are incredibly far off.
Thanks a lot!