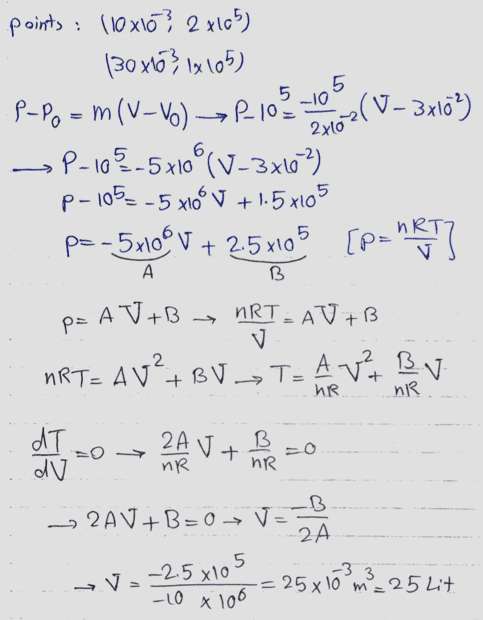Homework Help Overview
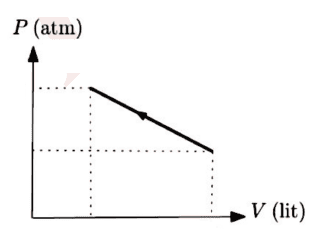
The discussion revolves around a diagram related to the condensation of an ideal gas, with participants questioning the accuracy of the diagram and the terminology used. There is a focus on understanding the implications of the diagram in the context of thermodynamics.
Discussion Character
- Exploratory, Conceptual clarification, Assumption checking
Approaches and Questions Raised
- Participants discuss the correctness of the diagram and whether it represents a maximum or minimum. There are questions about the appropriate terminology, specifically the use of 'condense' versus 'compress'. Some participants suggest using non-SI units for simplicity and explore the implications of the gradient of the line in the diagram.
Discussion Status
The discussion is active, with participants providing feedback on the diagram and terminology. Some guidance has been offered regarding the use of units and the need to verify whether the point in question is a maximum or minimum. There is an acknowledgment of the need for clarity in language and concepts.
Contextual Notes
Participants are navigating potential language barriers and the appropriateness of unit systems in the context of the problem. There is a mention of a derivative test being considered to analyze the maximum or minimum nature of the point in question.