Mcp
- 8
- 0
Consider any -R effect showing group like NO2 in place of anyone of the hydrogen of the benzene ring, here we get nitrobenzene.
We know that after showing -R effect a double bond will be formed between N and C and one of the π bond will no longer be present in the ring and it's place will be taken by + charge on ortho and para position which will be in conjugation with the remaining two π bonds in the ring as can be seen from the diagram below.
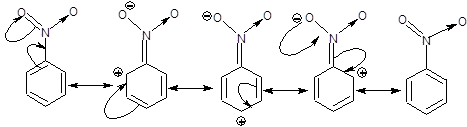
However the problem is that, in the canonical forms after resonance there will be 4 π electrons in a planar conjugated ring, which by definition is anti aromatic. But this should not be true because we know that resonance increases stability but anti aromatic hydrocarbons are unstable.
Can anyone give an explanation for the same.
We know that after showing -R effect a double bond will be formed between N and C and one of the π bond will no longer be present in the ring and it's place will be taken by + charge on ortho and para position which will be in conjugation with the remaining two π bonds in the ring as can be seen from the diagram below.
However the problem is that, in the canonical forms after resonance there will be 4 π electrons in a planar conjugated ring, which by definition is anti aromatic. But this should not be true because we know that resonance increases stability but anti aromatic hydrocarbons are unstable.
Can anyone give an explanation for the same.