- #1
Bolter
- 262
- 31
- Homework Statement
- See question below
- Relevant Equations
- F = mv^2/r
I am really stuck on what to do here in this question

I have arrived at forming an equation to work out the radius of electron orbit from doing the following
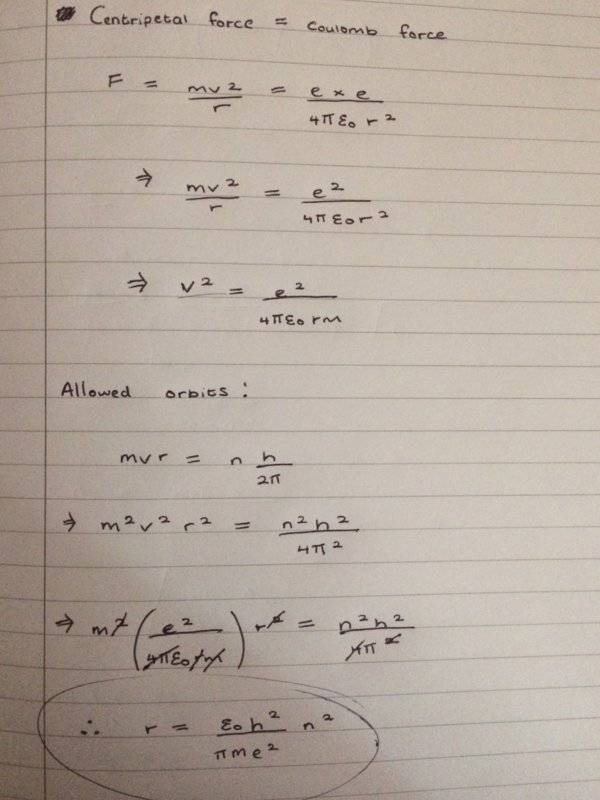
However I do not know what to do next as I don't know what the value of n (quantum number) must be?
Any help would be really great! Thanks
I have arrived at forming an equation to work out the radius of electron orbit from doing the following
However I do not know what to do next as I don't know what the value of n (quantum number) must be?

Any help would be really great! Thanks