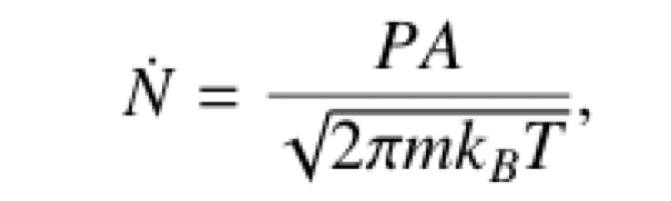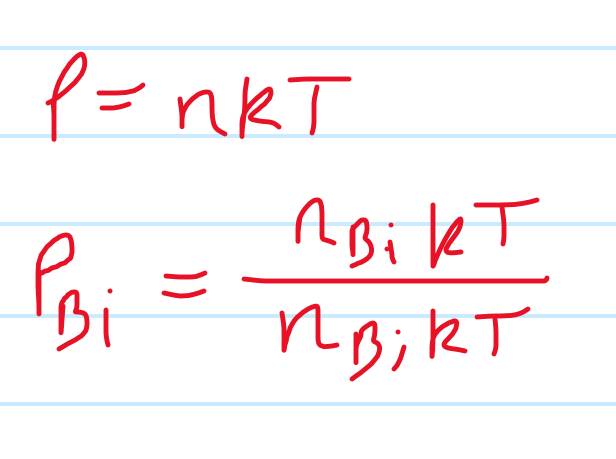so_gr_lo
- 69
- 10
- Homework Statement
- Bismuth is heated to 1100 K in an oven and gives rise to a beam of vapour molecules effusions from an aperture of area 10^-6 m^2. The beam contains Bi and Bi2 molecules in the ratio 1.41:1. Calculate the ratio of the partial pressures of Bi to Bi2 molecules
I believe the total pressure is the sum of the partial pressures but I’m not sure what to do with that, so I tried just calculating the ratio of the pressures but don’t know where to go from there
- Relevant Equations
- P=nKT where n is the number density per unit volume
and rate of effusion formula given below
Rate of effusion