Sabra_a
- 33
- 6
thread moved from a technical subforum
Summary: Hydrogen peroxide, H2O2, decomposes as per following reaction scheme:
H2O2(l) → H2O(l) + O2(g)
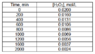
The concentration versus time data for the decomposition of hydrogen peroxide is given in Table 1.
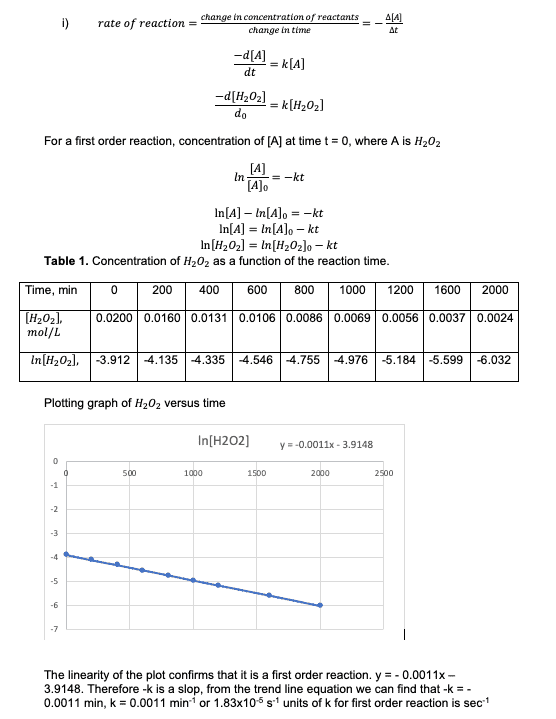
i) Calculate the reaction rate constant, k, graphically, with the aid of Excel software. Confirm that this is a first-order reaction.
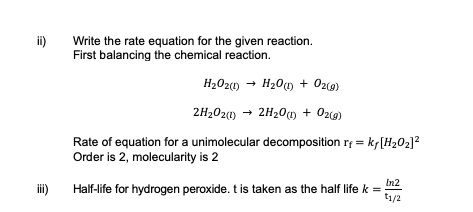
ii) Write the rate equation for the given reaction.
iii) Calculate the half-life for hydrogen peroxide.
I have attached the table and my attempt to solve the question, a feedback will be appreciated. I'm quite stuck on the 2nd and 3rd point
H2O2(l) → H2O(l) + O2(g)
The concentration versus time data for the decomposition of hydrogen peroxide is given in Table 1.
i) Calculate the reaction rate constant, k, graphically, with the aid of Excel software. Confirm that this is a first-order reaction.
ii) Write the rate equation for the given reaction.
iii) Calculate the half-life for hydrogen peroxide.
I have attached the table and my attempt to solve the question, a feedback will be appreciated. I'm quite stuck on the 2nd and 3rd point