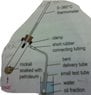
Discussion Overview
The discussion revolves around safety precautions in the fractional distillation of petroleum, particularly in a school laboratory setting. Participants explore the implications of using different heating methods and their potential risks.
Discussion Character
- Homework-related, Conceptual clarification, Debate/contested
Main Points Raised
- One participant notes that a small flame is recommended for heating rocksil soaked with petroleum to prevent breaking the test tube, but questions why a stationary flame should be avoided.
- Another participant highlights the risks associated with using a flammable distillate and small apparatus, suggesting that strong heating could lead to dangerous outcomes.
- A subsequent reply implies that burning could occur if the heating is too strong, but the conditions under which this might happen remain unclear.
- Further discussion contrasts the effects of strong heat versus gentle heat in the context of the small apparatus used.
Areas of Agreement / Disagreement
Participants express uncertainty regarding the specific dangers of using a stationary flame and the consequences of heating too strongly, indicating that multiple views and concerns remain unresolved.
Contextual Notes
The discussion does not clarify the exact mechanisms by which heating methods affect safety, nor does it address the assumptions underlying the recommendations for heating techniques.