- #1
pj33
- 24
- 3
- TL;DR Summary
- Explanation of lattice
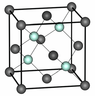
https://www.researchgate.net/figure/1-Silicon-crystallographic-structure-It-has-the-diamond-structure-which-is-two-fcc_fig4_34172659 the fcc silicon lattice is shown.
My question is:
Since the silicon atom has 4 valence electrons and requires 4 more to be completed, why are so many atoms shown in the lattice. There are 8 electrons shown as nodes and each of it, is connected to a neighbouring node, thus each silison requires one more to be complete.
Also, what is the difference between the blue-ish and grey atoms?
Sorry for this silly question, but I didnt study chemistry extensively in the past and I find some concepts hard to understand.
Thank you in advance
My question is:
Since the silicon atom has 4 valence electrons and requires 4 more to be completed, why are so many atoms shown in the lattice. There are 8 electrons shown as nodes and each of it, is connected to a neighbouring node, thus each silison requires one more to be complete.
Also, what is the difference between the blue-ish and grey atoms?
Sorry for this silly question, but I didnt study chemistry extensively in the past and I find some concepts hard to understand.
Thank you in advance