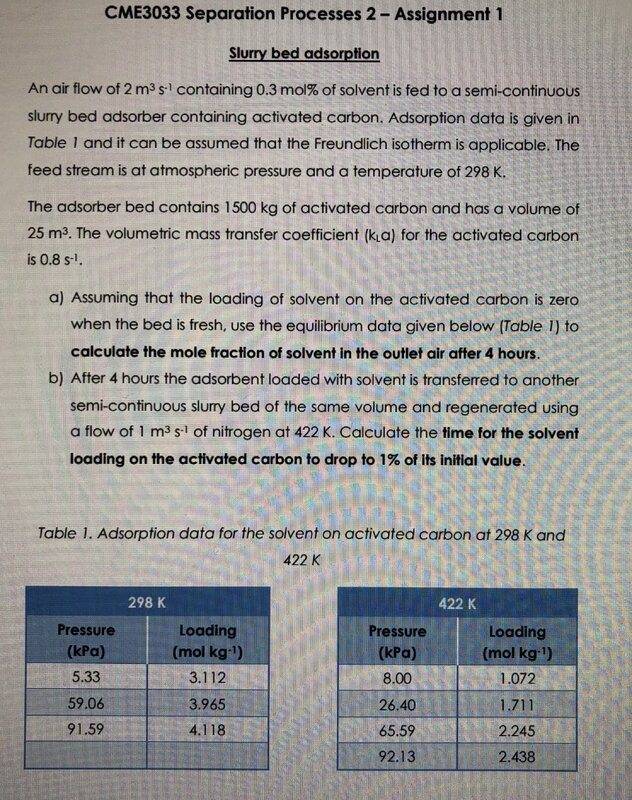
Will26040
- 21
- 3
Summary:: I have an assignment on slurry bed adsorption and would like to know how to calculate residence time as it is not specified. I was also wondering if anyone had any suggestions for another isotherm to try and fit the data to (other than freundlich). Thanks
##\frac {dq} {dt} = \frac {k_la .t_{res}} {1+k_la.t_{res}}.\frac QS.[c_F-c^*] ##
where:
##t_{res} =## residence time ##(s)##
## k_la =## volumetric mass transfer coefficient ##(s^{-1})##
##Q= ##Volumetric flowrate
##S= ##adsorbent mass
##c_F=## feed concentration
##c^*= ##Concentration in solution in equilibrium with the loading on the adsorbent (suitable isotherm used)
I know that I need to use the equation specified above and solve numerically to get the loading after 4 hours. However, I am not sure how to get the residence time, as this has been specified in previous examples that I have done. I was also wondering if anyone had any suggestions for another isotherm to try and fit the data to (other than freundlich). Thanks
##\frac {dq} {dt} = \frac {k_la .t_{res}} {1+k_la.t_{res}}.\frac QS.[c_F-c^*] ##
where:
##t_{res} =## residence time ##(s)##
## k_la =## volumetric mass transfer coefficient ##(s^{-1})##
##Q= ##Volumetric flowrate
##S= ##adsorbent mass
##c_F=## feed concentration
##c^*= ##Concentration in solution in equilibrium with the loading on the adsorbent (suitable isotherm used)
I know that I need to use the equation specified above and solve numerically to get the loading after 4 hours. However, I am not sure how to get the residence time, as this has been specified in previous examples that I have done. I was also wondering if anyone had any suggestions for another isotherm to try and fit the data to (other than freundlich). Thanks